20
Specifications
Technical data
Product name : Omron Ultrasonic Nebuliser
Model : NE-U17 (NE-U17-E)
Power source : 230 VAC, 50 Hz
Power consumption : Approx. 80 VA
Fuse : T2A L250V
Ultrasonic frequency : Approx. 1.7 MHz
Particle size : * MMAD 4.4 μm (MMAD=Mass Median Aerodynamic Diameter)
Nebulisation rate : ** Approx. 0 to 3 ml adjustable
Sound : ** Less than 45 dB
Air volume : Maximum of 17 l/min
Amount of cooling water : Approx. 375 ml
Capacity of medication cup : Approx. 150 ml (min. 5 ml)
External dimensions : Approx. 276 (W) x 243 (H) x 226 (D) mm
Weight of the main unit : Approx. 4.0 kg
Protection class : Class I
Applied parts class : Type B
Operating condition : Continuous
/PERATING TEMPERATURE # TO # & TO & TO 2(
humidity
3TORAGE TEMPERATURE # TO # & TO & TO 2( TO H0A
humidity / air pressure
Accessories included : Inhalation hose M (with a cuff, 70 cm), Mouthpiece set, 2 Medication cups,
Power cord, Instruction manual (with warranty card)
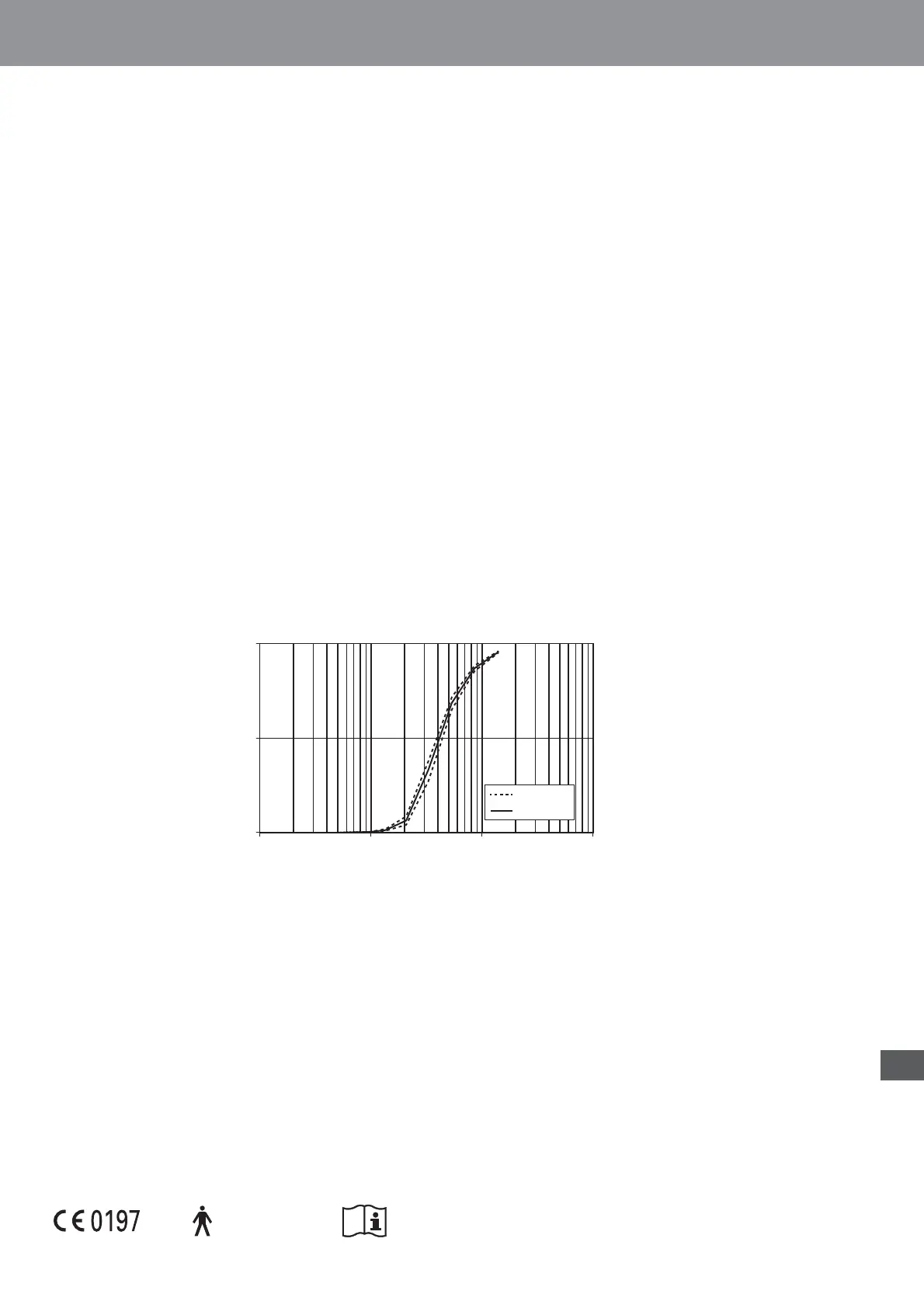
Result of cascade impactor measurements for particle size
0
50
100
0.1 1 10 100
individual
mean
#UMULATIVE PARTICLE MASS OF SODIUM FLUORIDE UNDER SIZE
Dp (μm)
#UMULATIVE 5NDERSIZE
* Independently measured at SolAero Ltd., Canada, Dr. John Dennis, according to EN13544-1:2007+A1:2009.
** Measured by OMRON HEALTHCARE Co., Ltd.
Notes:
s 3UBJECT TO TECHNICAL MODIFICATION WITHOUT PRIOR NOTICE
s 4HIS /-2/. PRODUCT IS PRODUCED UNDER THE STRICT QUALITY SYSTEM OF /-2/. (%!,4(#!2% #O ,TD *APAN
s 4HE DEVICE MAY NOT WORK IF THE TEMPERATURE AND VOLTAGE CONDITIONS ARE DIFFERENT TO THOSE DEFINED IN THE SPECIFICATIONS
s $O NOT USE THE DEVICE WHERE IT MAY BE EXPOSED TO FLAMMABLE GAS
s 4HE .EBULISATION RATE IS MEASURED WITH SALINE SOLUTION AT # AND CAN VARY WITH MEDICATION AND AMBIENT CONDITIONS
s 4HE DISTRIBUTION OF PARTICLE SIZE IS MEASURED WITH .A& SOLUTION AND CAN VARY WITH MEDICATION AND AMBIENT CONDITIONS
s 0ERFORMANCE MAY VARY WITH DRUGS SUCH AS SUSPENSIONS OR HIGH VISCOSITY 3EE DRUG SUPPLIERS DATA SHEET FOR FURTHER DETAILS
s 3EE WEB SITE OF /-2/. (%!,4(#!2% %52/0% TO UPDATE TECHNICAL INFORMATION
URL: www.omron-healthcare.com
s 4HIS DEVICE FULFILS THE PROVISIONS OF THE %# DIRECTIVE %%# -EDICAL $EVICE $IRECTIVE AND THE %UROPEAN 3TANDARD
EN13544-1:2007+A1:2009, Respiratory therapy equipment - Part1: Nebulising systems and their components.
Symbols:
=Type B
Read the instruction
manual carefully
EN
 Loading...
Loading...











