REBOTEC / BA-Nr.: 02.09.200/2021-08-A01
- 4 -
2.5. Packaging
• We recommend you keep the packaging for a later
transport.
• Dimensions : 1240mm x 350mm x 690mm
2.6. Disposal
• Disposal and recycling of used products and pack-
aging must be carried out in accordance with cur-
rently valid regulations. Please contact a disposal
company for further information.
• The Infection Protection Act must be observed.
• Please observe any labels and information on
packaging material and act accordingly.
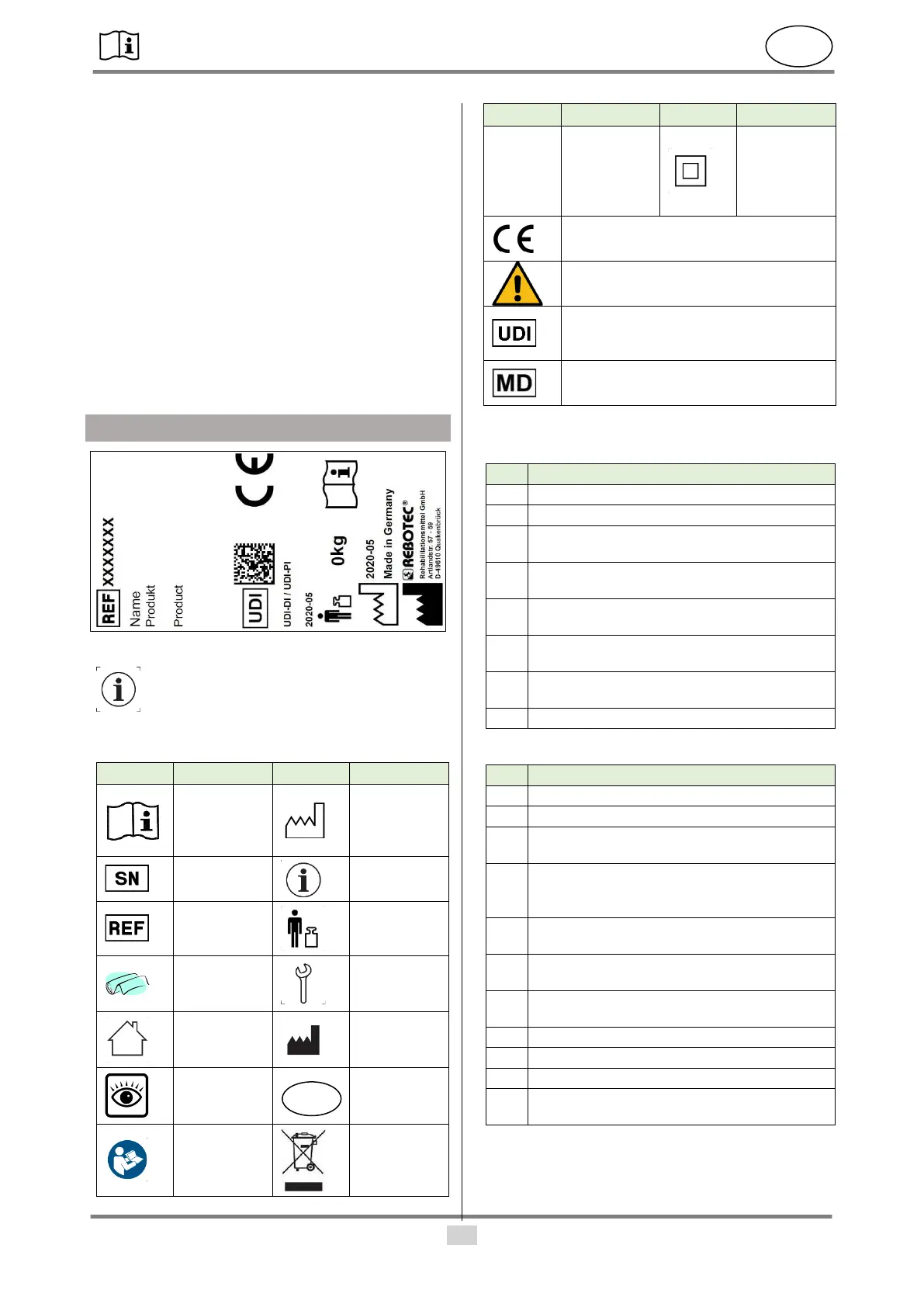
3. Symbols & nameplates
Figure similar.
The nameplate must not be removed!
For ordering spare parts and processing
complaints, the device name, serial number and
year of manufacture will be required.
Year of
manufac-
ture of De-
vice
Maximum
user weight
in kg
Mainte-
nance in-
structions
Applicable
for indoor
use only.
Do not dis-
pose of as
household
waste.
Degrees of
protection
according
to DIN EN
60529
Product complies with (EU) 2017/745
MDR
Caution!
Observe the safety instructions
Unique Device Identifier
(Data: GTIN / LOT (SN) / year of man-
ufacture)
Medical device label
This product is a medical device.
3.1. Degrees of protection
First digit:
Protected against solid foreign bodies with
a diameter ≥ 50 mm
Protected against solid foreign bodies with
a diameter ≥ 12.5 mm
Protected against solid foreign bodies with
a diameter ≥ 2.5 mm
Protected against solid foreign bodies with
a diameter ≥ 1.0 mm
Protected against dust in harmful quanti-
ties
Protection against vertically falling drops
of water
Protection against vertically falling drops
of water when the enclosure is tilted up to
15°C
Protection against water falling as a spray
at any angle up to 60° from the vertical
Protection against water splashed from
any direction
Protection against water jets (nozzle) from
any angle
Protection against powerful water jets
Protection against temporary immersion
Protection against continuous immersion
Protection against water from high-pres-
sure/steam jet cleaning

 Loading...
Loading...