Cosmetic Care & Maintenance
7-25
Galvanic/Stray Current Corrosion
Metal parts underwater can be subjected to two basic styles of
electrolysis: galvanic corrosion and stray current corrosion. Both can
damage the drive, propeller, underwater parts, boat and motor if not
correctly monitored (testing at 2 week intervals) and avoided.
Galvanic corrosion is an electrochemical reaction between two or
more metals. Drive systems consist of several different metals. Some
are more active than others.
Galvanic corrosion of the more chemically active metals can occur
whenever two or more dissimilar metals that are “grounded” (connected
by actually touching each other, or through a wire or metal part) are
immersed in a conductive solution (any material that can conduct
electricity). Anything but pure water is conductive. Saltwater, fresh
water with a high mineral content and polluted freshwater are highly
conductive. Conductivity increases with temperature. That is why
Florida boats experience more corrosion than boats in Maine.
Specifi cally look at a typical marine drive unit with a stainless steel
propeller. The aluminum is the more chemically active metal (called
the anode) and the stainless steel propeller is the less chemically active
metal (called the cathode).
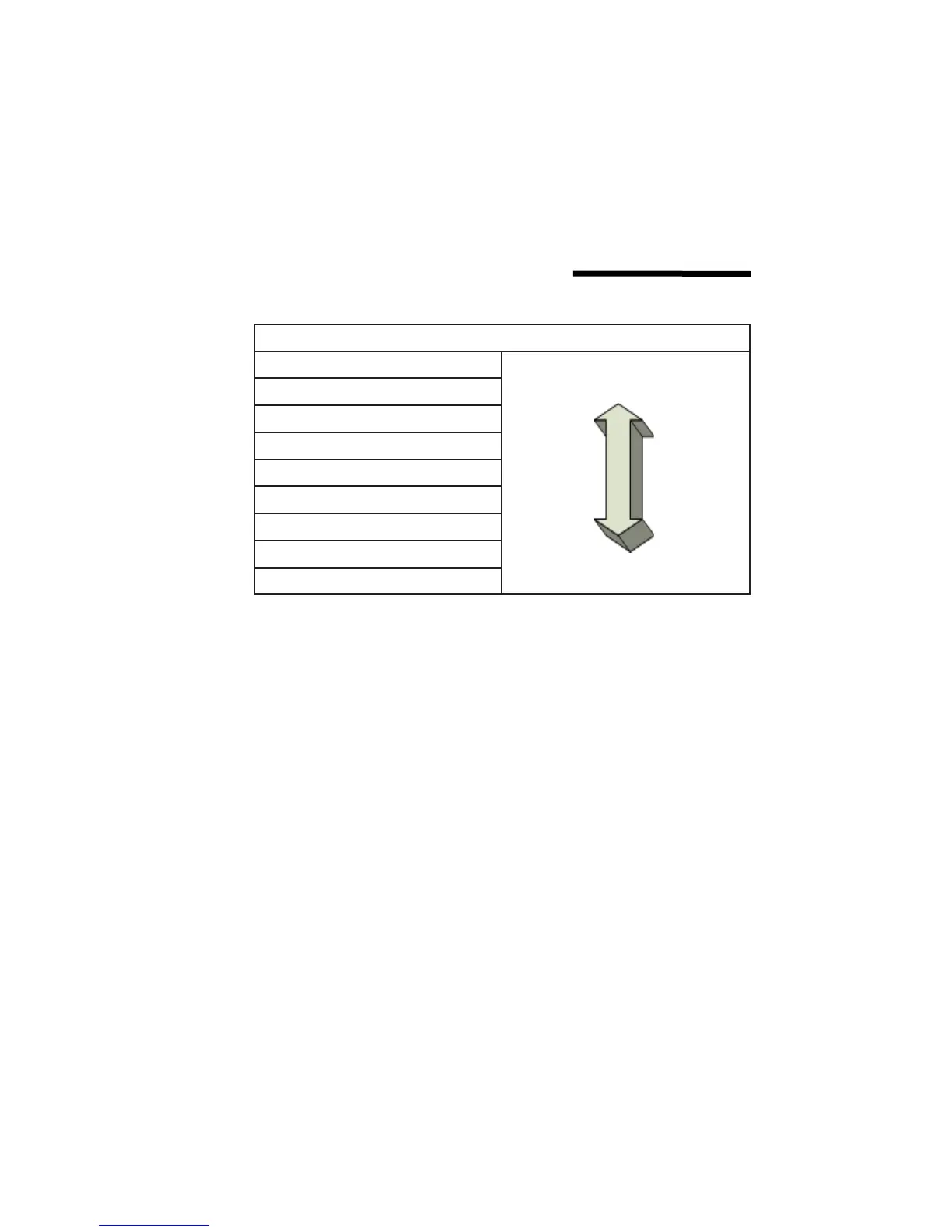
CORROSION TABLE
Gold
Stainless Steel
Bronze
Copper
Brass
Steel
Aluminum
Zinc
Magnesium
Least Active
Most Active
 Loading...
Loading...