English 1
Disinfection and sterilization guide Clinical use only
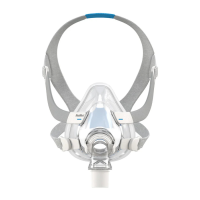
This guide is intended for multipatient use of the AirFit
™
F20 / AirTouch
™
F20 ResMed full face mask in a sleep lab, clinic or hospital. If you use the mask as a single user in the home, refer to the User
Guide for cleaning instructions. This guide describes ResMed’s recommended and validated procedures for cleaning, disinfection and sterilization of the mask in accordance with ISO17664.
AirFit F20 / AirTouch F20
mask component
1
High level thermal disinfection High level chemical disinfection Sterilization Validated number of cycles
2
167°F (75°C)-30 min;
176°F (80°C)-10 min;
194°F (90°C)-1 min
200°F (93°C)-10 min CIDEX
™
OPA
Ortho-phthalaldehyde 0.55%
STERRAD
™
100S STERRAD
™
NX
Standard or
Advanced cycle
• AirFit F20 cushion 30
• AirTouch F20 cushion
3
— — — — —
N/V
4
• Elbow
— — —
30
• Frame
— — —
30
• Headgear
— — — —
30
1
This mask may not be available in all regions. For full details regarding the correct use of this mask, please refer to the specific User Guide. For a list of available replacement parts for each mask system, check the Components Card on www.resmed.com.
2
If a healthcare facility requires an additional disinfection or sterilization cycle after reassembly, the number of validated cycles must be halved.
3
The AirTouch F20 cushion is intended for single-patient reuse in both home and hospital/institutional environments and recommended to be replaced monthly.
4
Not validated.
A
1
2
3
4
B C
5 6 7
D
A Elbow
1 Valve (AAV)
2 Vent
3 Side buttons
4 Swivel
B Frame
C Cushion
D Headgear
5 Magnetic clips
6 Lower headgear straps
7 Upper headgear straps
A+B+C Frame system
A+B+C+D Complete system










 Loading...
Loading...