SD CodeFree™ BLOOD GLUCOSE MONITORING SYSTEM
78
Product Technical Information
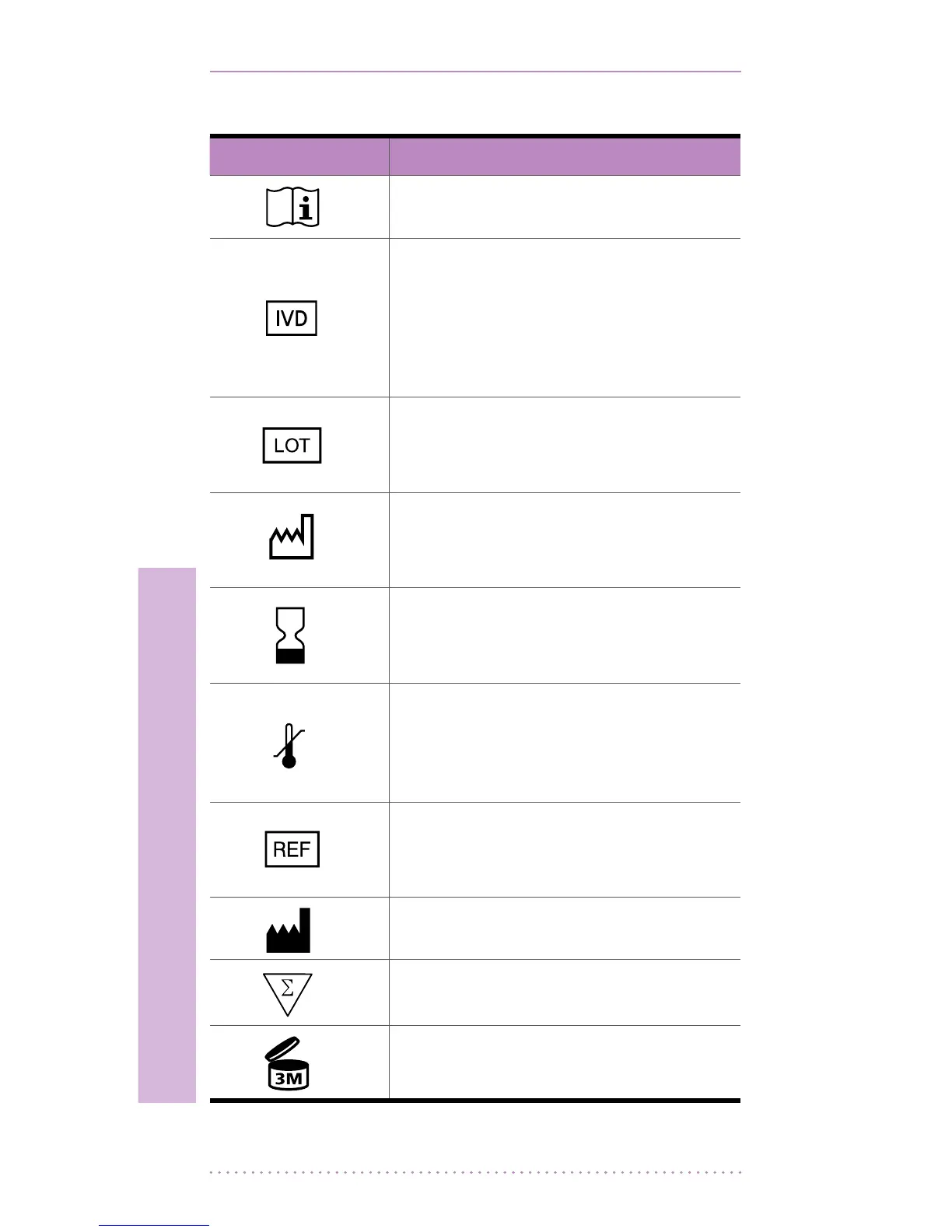
2. Package Symbols for the BGM system
Symbol Description
Consult instructions for use
IN VITRO DIAGNOSTIC MEDICAL
DEVICE:
This system is intended for
use outside the body (in vitro
diagnostic use).
BATCH CODE:
To indicate the lot number for this
system.
DATE OF MANUFACTURE:
To indicate the date of
manufacture for this system.
USE BY:
This item should be used by the
given date.
To indicate the temperature
limitations in which the transport
package has to be kept and
handled.
CATALOGUE NUMBER:
To indicate the catalogue number
for this system.
To indicate the manufacturer.
ContainsSucientfor<n>Tests.
Use for a maximum of 3 months
after rst opening the container.
 Loading...
Loading...