Section 2: Precision & Accuracy Name
Date
Fundamental Topics in Science © 2001 Texas Instruments
Try-It!™ 2-5
Calculate percent error
If the accepted value for the mass of 125.0 milliliter of water is 125.0 g, how accurate would you
consider this set of measurements? You can express the accuracy in terms of percent error.
percent error =
(accepted value
1
experimentally determined value)
(accepted value)
g
100
Use your TI
1
83 Plus to calculate the percent error.
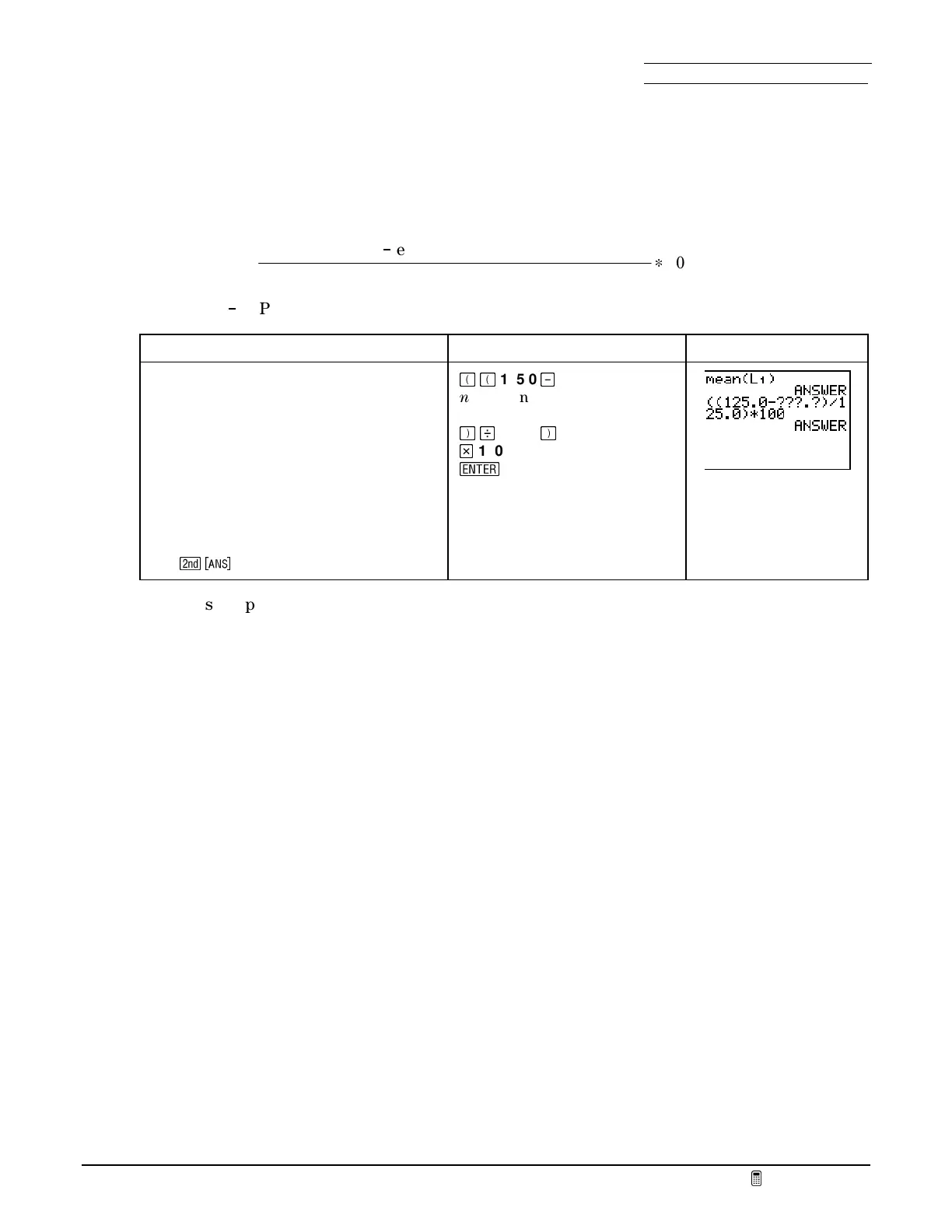
To Do This Press Display
1. Calculate the percent error.
Parentheses are very important for
correctly grouping numbers in
calculators.
Remember to retain only four
significant figures in the mean.
Tip:
Instead of retyping the answer from
a previous calculation, you can press
\
=
.
125.0
nnn.n
(enter the mean
calculated above)
125.0
100
b
What was the percent error? Do you think this is a large error or a small error?
#

 Loading...
Loading...