DOC-00322-ENA.doc Page 3 of 29 ! Wescor, Inc
1 Introduction
This document contains the information required by the In Vitro Diagnostic Directive (98/79/EC) Annex I (Essential
Requirements), Part B, Section 8 (Information supplied by the manufacturer) for the Aerospray
"
AFB Stainer/Cytocentrifuge
(Model 7720), its accessories and supplies. In particular, it describes any symbols used on labels and on the instrument, hazards
associated with the stain reagents used, the intended purpose of the device, lot numbers and expiration dates, and instructions
for the use and maintenance of the device.
Some sub-requirements of Section 8 are not applicable to this product, but the applicable requirements are referenced herein.
This document is available in the official language of each EC member state where the product is sold that requires information
in its own language. Additional helpful information may be found in Wescor User’s Manuals, Service Manuals, Technical
Bulletins, or other information supplied by Wescor or its Authorized Distributors for specific countries. Some supplementary
material is in the English language only. Many of these materials may be found on Wescor’s web site: www.wescor.com
. A
Document Packet is included with each Aerospray
"
AFB Stainer/Cytocentrifuge, which includes MSDS sheets, a Declaration of
Conformity, Nozzle Cleaning Instructions, an Installation Checklist, and a User’s Manual (8.1).
Aerospray
"
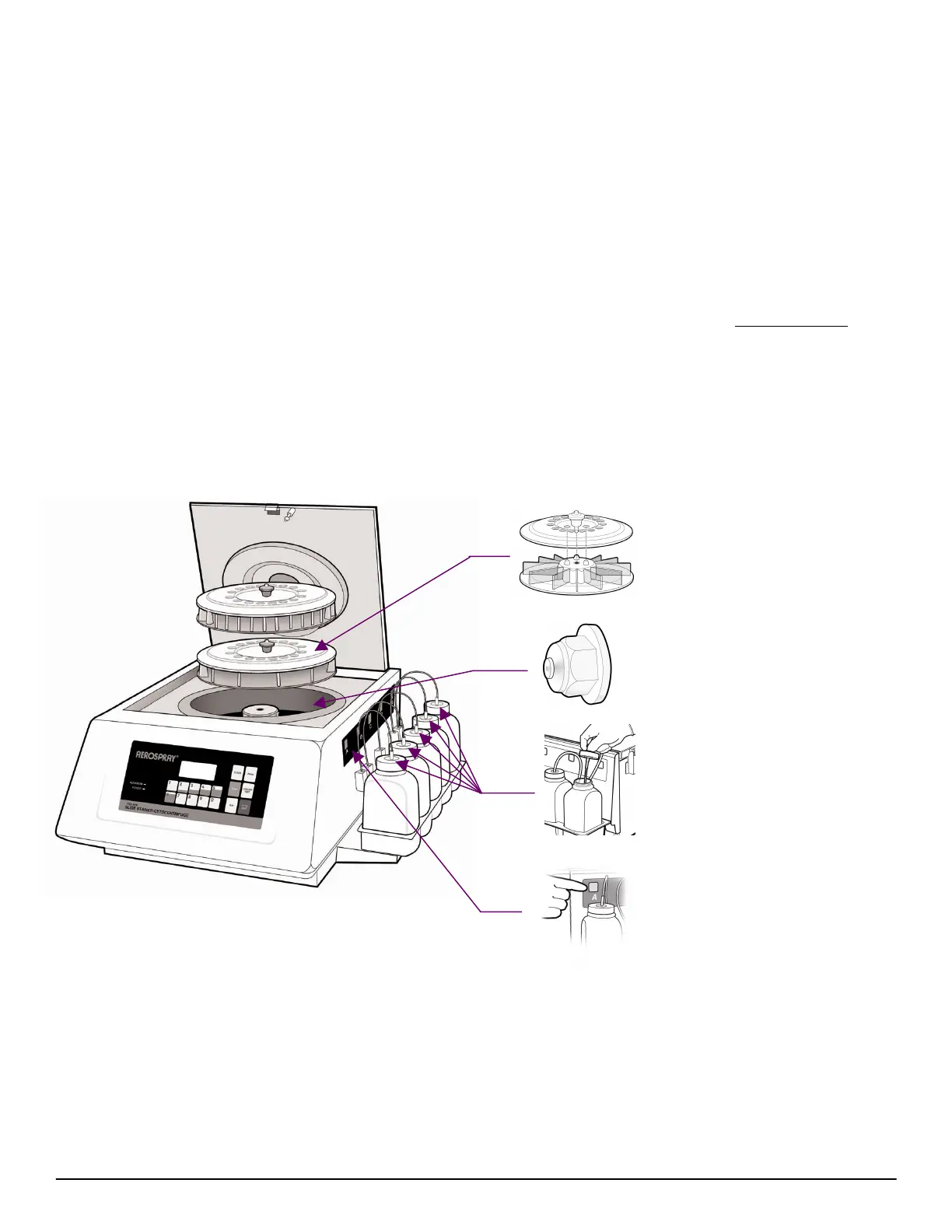
AFB Slide Stainer/Cytocentrifuge Front and Side
Slide Staining Carousels
The carousel hold 1 to 12, or 1 to
30 slides. It mounts on the drive
hub, rotating at approximately 20
rpm for staining and approximately
950 rpm for drying.
Reagent Spray Nozzle
Each reagent has a separate spray
nozzle(s) to dispense the correct
amount of reagent.
Reagent Bottle Dip Tubes
Five reagent dip tubes, A through
E, connect the reagents to the
internal pumps and spray nozzles.
Manual Priming Buttons
These buttons operate the
corresponding pumps for priming.
 Loading...
Loading...