011807-EN--B (2022-12_S617 S717F S900 S950 C50_IFU)
40 / 49
WARNING: Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in
improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they
are operating normally.
WARNING: Portable RF communications devices (including peripherals such as antenna cables and external antennas) should
not be used closer than 30 cm (12 inches) to any part of the medical device, including specified cables by the manufacturer.
Otherwise, the performance of these devices could be impaired.
8.4 ELECTROMAGNETIC EMISSIONS
The medical device is designed for use in the electromagnetic environment described in the table below. The user and/or
installer must therefore ensure that the medical device is used in the environment described below.
Electromagnetic environment - comments
Conducted disturbances
(conducted emissions)
CISPR 11
The medical device uses RF energy for its internal functioning.
Electromagnetic radiation
disturbance
(Radiated emissions)
CISPR 11
Harmonic current emissions
IEC 61000-3-2
Professional healthcare facility environment and home healthcare
environment.
Voltage changes, voltage
fluctuations and flicker
emissions
IEC 61000-3-3
The medical device is intended for use in a professional healthcare environment (hospital, clinic) and in a home healthcare
environment (dental office in a residential area).
The user and installer should therefore ensure that the medical device is used in the environment described below.
For the professional healthcare environment, the medical device should not be used in the vicinity of electrosurgery equipment
or in the vicinity of an electromagnetically shielded room for magnetic resonance imaging (MRI) equipment where the intensity
of electromagnetic disturbances is high.
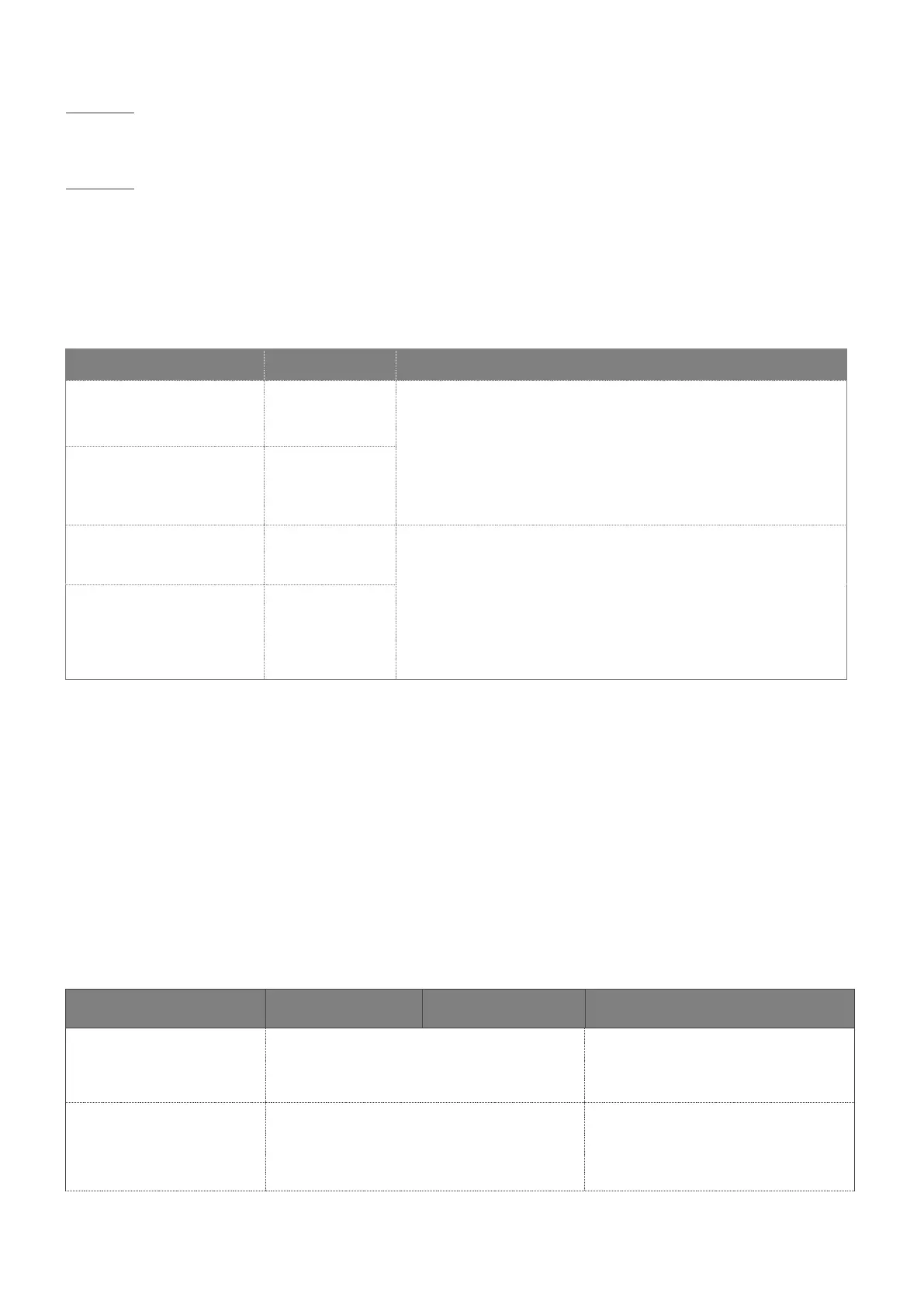
8.5 MAGNETIC AND ELECTROMAGNETIC IMMUNITY
The medical device is designed for use in the magnetic and electromagnetic environment described in the table below. The
user and/or installer must ensure conformity of the electromagnetic environment.
Test level
(IEC 60601-1-2)
Electromagnetic environment -
comments
Electrostatic discharge
immunity
IEC 61000-4-2
± 8 kV contact
± 2 kV, ± 4 kV, ± 8 kV, ± 15 kV air
Professional healthcare facility
environment and home healthcare
environment.
Radiated RF electromagnetic
field immunity
IEC 61000-4-3
10 V/m
80 Hz – 2,7 GHz
80 % AM at 1 kHz
Professional healthcare facility
environment and home healthcare
environment.
 Loading...
Loading...