11
d. Technical characteristics
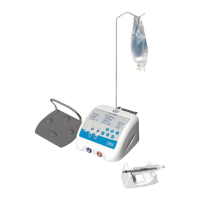
Console / micro motor / footswitch
40000 rpm / Torque: 28.6
mN.m
IIa rule 9 in Annex IX 93/42/EEC
Protective index
micro motor
Protective index
footswitch
Not suitable for use in presence of flammable anesthetics or oxygen.
Conditions of use: Temp +18°C/+40°C (+64°F/+104°F) RH < 80%. Altitude restriction:
3.000m (10.000 ft)
Shipping and Storage conditions: Temp +5°C/+65°C (+41°F/+149°F) RH < 20-95% non condensing.
IV– INSTALLATION / FIRST USE
4.1 UNPACKAGING THE DEVICE
Upon reception of the device, check for any damage caused in transit.
Contact your supplier if necessary.
4.2 RECOMMENDATIONS
Have your device connected to the power supply by a certified dental installation technician. The electrical
connection of the I-SURGE must comply with the standards applicable in your country.
4.3 INSTALLATION
Important: Do not place the I-SURGE close to or on top of another device.
Do not place the power cord and the footswitch cord in a wire cover or in a cable gland.
 Loading...
Loading...