Wet Bath Simulator Methodology
Dräger Alcotest 9510 Washington Technical Manual V3.1 2014 9
current between the two platinum plates, the current of which
is measured. This becomes the usable indicator of the amount
of ethanol consumed by the fuel cell, and is directly
proportional to the ethanol concentration of the breath sample.
After processing, a quantitative result is determined. A rise in
BrAC will result in a proportionate increase in voltage.
Other alcohols will react in the cell, but because the chemistry
is d
if
ferent, the rate of reaction is also different (e.g.
Isopropanol and Methanol).
6.4 Fuel Cell Analytical System of the 9510
The fuel cell contained within the instrument is located directly
on top of the cuvette and therefore heated by the cuvette.
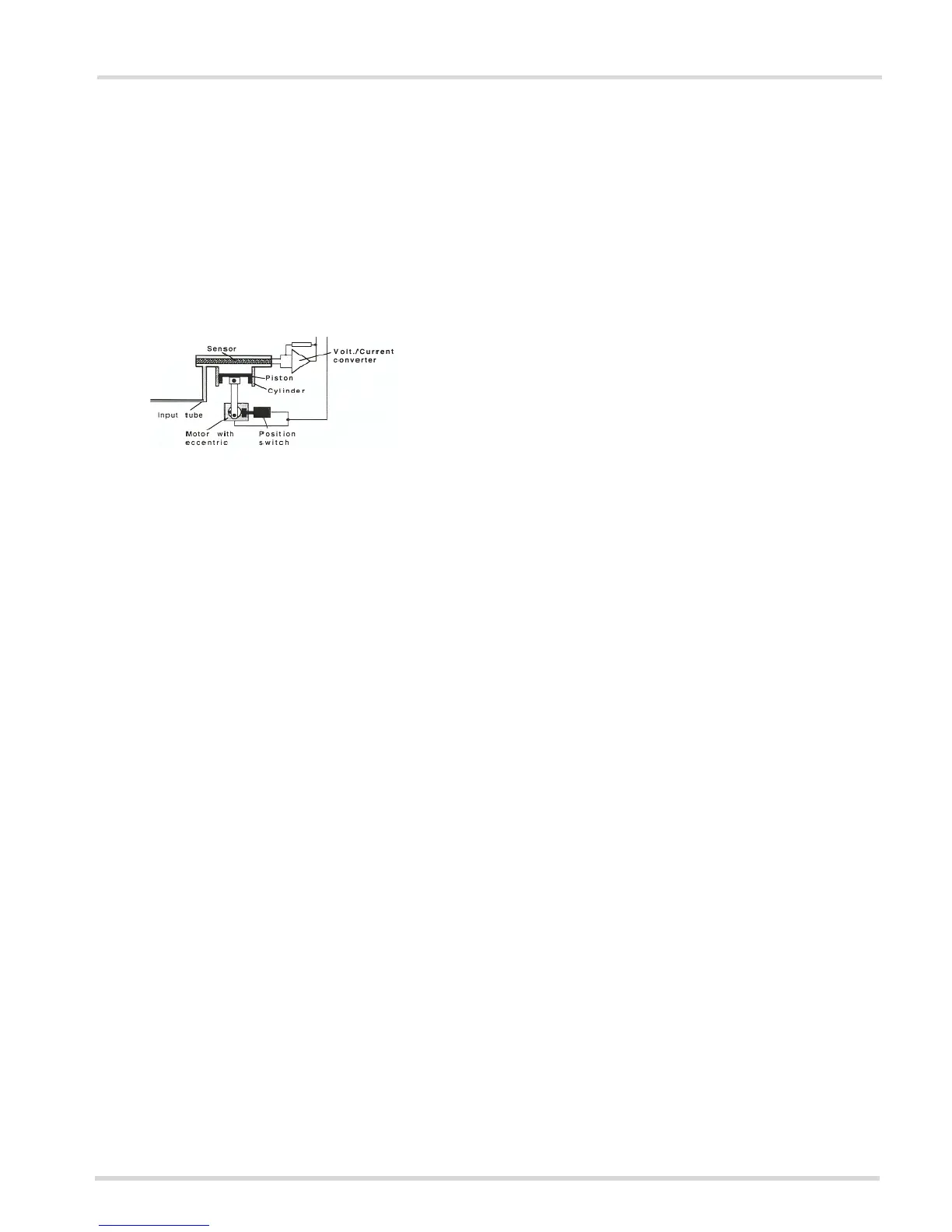
The fuel cell sampling system consists of:
Fuel Cell: An alcohol specific sensor.
Piston: Draws a 1cc sample out of the cuvette and into the
fuel cell.
Cylinder: The housing for the piston.
Breath Inlet Pathway: Allows the breath sample to pass
from the breath hose to the cuvette.
Motor: Drives the piston.
Position Switch: Indicates that the motor has completed
its cycle.
6.4.1 Electrochemical Sensor
Measures small samples from inside the c
uvette. Once
etha
nol reaches the sensor, a chemical reaction is triggered.
The resulting current is used to determine the amount of
alcohol in the sample.
6.5 Benefits of Dual Sensing Technology
By combining two distinct analytical systems to analyze a
subject’s breath, the 9510 is able to provide two precise,
accurate, and independent test results.
Infrared spectroscopy requires that a zero reference be
est
ablished
prior to a subject’s breath test. Because the fuel
cell of the 9510 is “piggy backed” on the IR cuvette, the 9510
can draw a sample out of the chamber and analyze it, to
ensure that a zero set is based on one that is free of absorbing
alcohol vapor.
The dual system also allows for a greater degree of sensitivity
to any po
ssible existence of interfering substances. Because
the fuel cell is alcohol specific, and the IR sensor operates at
9.5µm in the IR spectrum, the possibility of an interfering
substance influencing a subject’s ethanol reading is virtually
impossible.
To quote A.W. Jones in his article, “Measuring Alcohol in Blood
and Brea
th for Forensic Purposes - A Historical Review”:
“The use of a higher wavelength (9.5µm) offered the
advantage
that the results were much less prone to
interference from acetone and hydrocarbons which absorb IR
radiation at 3.4µm and under some circumstances might be
expelled in the breath. In the latest generation of evidential
breath alcohol instruments, IR and electrochemical detectors
are contained within the same unit (Alcotest® 9510). As
mentioned earlier, analyzing alcohol by two independent
methods is highly desirable feature for medicolegal purposes.”
7 Wet Bath Simulator Methodology
Using wet bath simulators for calibration testing has been the
standard method for decades. Breath alcohol simulators are
specially designed instruments which provide equilibration of
alcohol between water and air at a controlled temperature.
The water-alcohol solution is maintained at a temperature of
34°C. The alcohol concentration of the vapor produced by a
wet bath simulator is proportional to the alcohol concentration
of the alcohol-water solution at a constant temperature (34°C)
in accordance with Henry’s Law.
7.0.1 Henry’s Law
Henry, an English chemist, studied the behavior of solutions in
which a volatile substance (one which readily evaporates to
form a gas) was dissolved and in 1803 he described the
behavior as a law now known as Henry’s Law. Although Henry
did not study alcohol solutions in his work, his law applies to
aqueous (water) solutions of alcohol containing less than 20
percent alcohol.
When a volume of alcohol is added to water it dissolves to form
a sol
ution. If an al
cohol solution is poured into a bottle (so as
to partially fill it), and the bottle is sealed, the concentration of
alcohol vapor in the air (and water vapor) above the solution
increases rapidly until it reaches a certain concentration and it
then remains at this concentration. At this stage, there will be
a definite ratio between the concentration of alcohol in the
solution and that in the air. The concentration of the alcohol
vapor above the solution depends on two factors: the
temperature of the system and the concentration of alcohol in
the solution.
From these observations a simplified version of Henry’s Law
may be st
ated:
“When an
aqueous solution of a volatile compound comes to
equilibrium wi
th air, there is a fixed ratio between the
concentration of the compound in air and its concentration in
the solution and this ratio is constant for a given temperature.”

 Loading...
Loading...