ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
LABELS
The device is manufactured in accordance to the Good Manufacturing Practices set forth by
the FDA, ISO Standards (International) and CE (Conformité Européenne or European
Conformity) standards and testing results per Article 9. The device is a Class I Shock
Protection and a Class II Medical device. Each of these governing agencies requires specific
labeling. All required labels are affixed per the relevant codes. Each label is pictured and
described in this section. Additionally, the placement of each label on the device is
communicated.
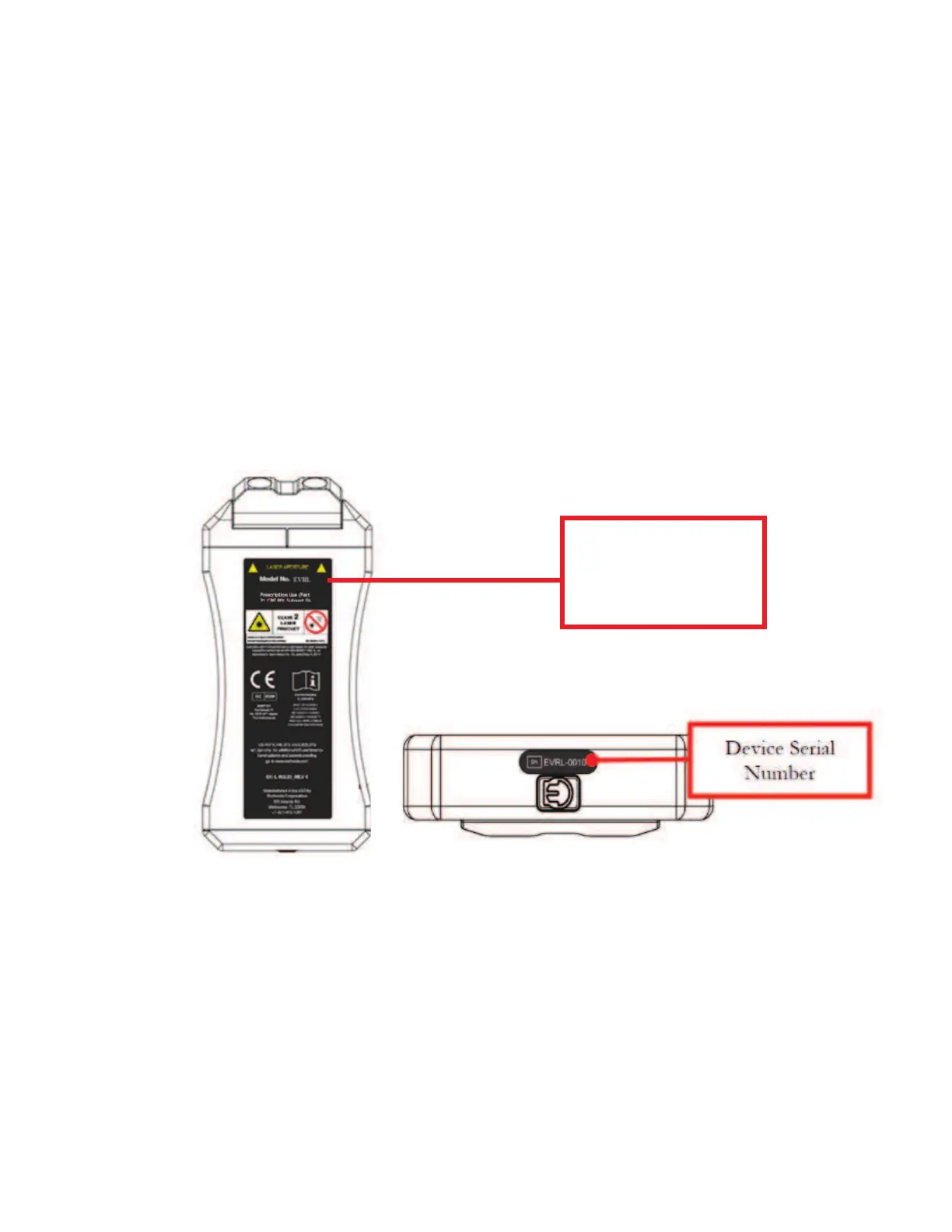
The following diagram shows the compliance label, device serial number and their placement.
The large black background label is this primary label and is compliant to FDA and ISO
standards; the image captures the FDA code regulated classifications and International
criteria.
Compliance
Label

 Loading...
Loading...