999075 – Rev F Page 9 of 40
1 . 7 Electromagnetic Compatibility (EMC) Statement
The following statement has been made against the assumption that the user of the system utilises the
provided components supplied by the manufacturer of the device to operate the device as intended. DO NOT
use any other form of power charge with the system as the manufacturer’s adapter has been assessed and
complies with the EMC requirements.
This product, has been designed, manufactured, and tested in accordance with the legal requirements for the
environment in which the device will be used within.
Pacemakers, defibrillators, and other medical devices should be manufactured in such a manner that they can
withstand Electromagnetic Interferences (EMI) in accordance with their associated mandatory European
directives and regulations. Please consult the user alert card which would have been issued to the user
regarding the use of electrical items for those individuals fitted with these or any other devices.
If users of this equipment are unsure of its compliance to EMC, you can request the confirmation from the
manufacturer that the product is manufactured to the appropriate Electromagnetic Compatibility standard.
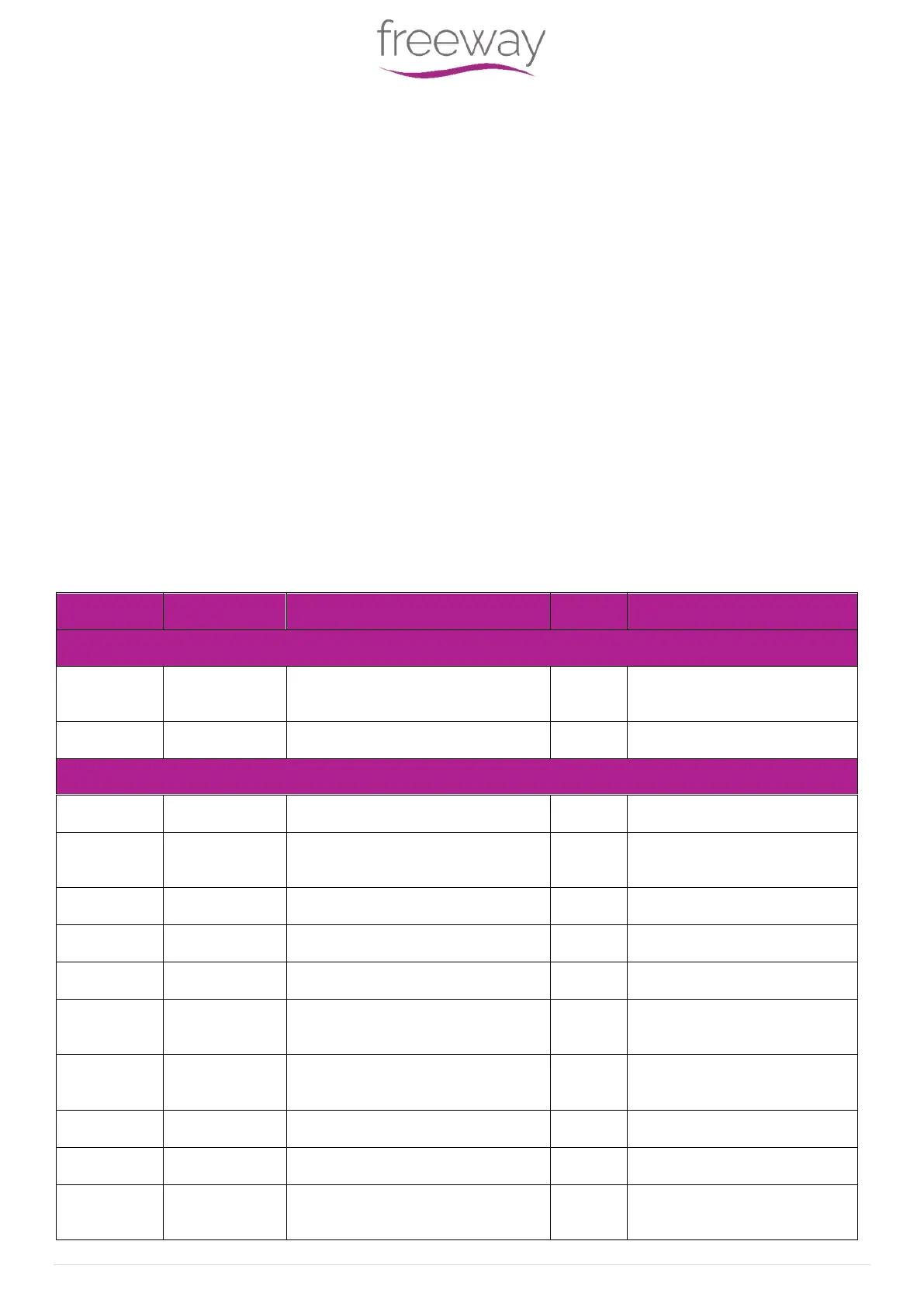
A brief summary of the tests carried out in accordance with IEC 60601-1-2 is shown in the table below.
The Hoist is also classified as Class B according to CISPR 11:2009 for the home health care environment.
The use of the device within the correct area where the intended use is given will have no detrimental effect on
other devices that have been tested to their intended respective requirements.
 Loading...
Loading...