6
9. DISPOSAL
Dispose of the battery and all components of the curing
light according to local regulations.
10. WARRANTY / REPAIR
Warranty: 3 years from the date of purchase for the
handpiece, electronic module, charging station and
power supply.
In case of a breakdown during the term, repair will be
carried out free of charge provided that the unit has been
used under normal conditions and according to the
instructions for use.
Consumables (such as light guide and eye-protection
shields) are not warranted.
The battery is a consumable, but has a 12 month warranty
applicable only to battery failure.
In order to benefi t from the warranty service, the
customer must return the apparatus to be repaired to the
GC Europe dealer/importer from which it was purchased,
at his own expense.
Before returning the device, please ensure that the
products are fully decontaminated and free of debris and
other organic materials such as blood residues, saliva and
other bodily fl uids. Contaminated devices will not be
repaired/replaced, and the cost for decontamination of
the environment aff ected by the device will be charged
on the basis of the actual cost of the materials and labor
required to decontaminate.
The apparatus should be returned suitably packed
(possibly in its original packing material), accompanied by
all the accessories and by the following information:
a) Owner’s details, including his telephone number.
b) Details of the dealer/importer.
c) Photocopy of the consignment note/purchase invoice of
the apparatus issued to the owner and indicating, in
addition to the date, also the name of the apparatus and
its serial number.
d) A description of the problem.
Transport and any damages caused during transport are
not covered by the warranty.
In the event of failures due to accidents or improper use,
or if the warranty has lapsed, repairs to the products will
be charged on the basis of the actual cost of the materials
and labour required for such repairs.
11. PACK AGING
Contents of the kit:
Handpiece sleeve 1
Electronic module 1
Battery packs 2
8mm black light-guide (120°) 1
Charging station 1
Power supply 1
EU plug adapter 1
UK plug adapter 1
Soft eye-protection shields 3
Hard, oval eye-protection shield 1
Accessories available separately:
Handpiece sleeve, light-guide, battery pack, charging
station, power supply with adapters, hard eye-protection
shield, soft eye-protection shields (x10)
12. EQUIPMENT CLASS
The product complies with all the provisions of the 93/42/
EEC Directive on medical devices (as amended by the
2007/47/EC Directive) and of the 2011/65/EU Directive on
the restriction of the use of certain hazardous substances
in electrical and electronic equipment that apply to it.
Classifi cation of product:
Class I medical device according to Rules 5 and 12 of
Annex IX of the 93/42/EEC Directive.
Applied part Type BF
Protection from liquids IP XO
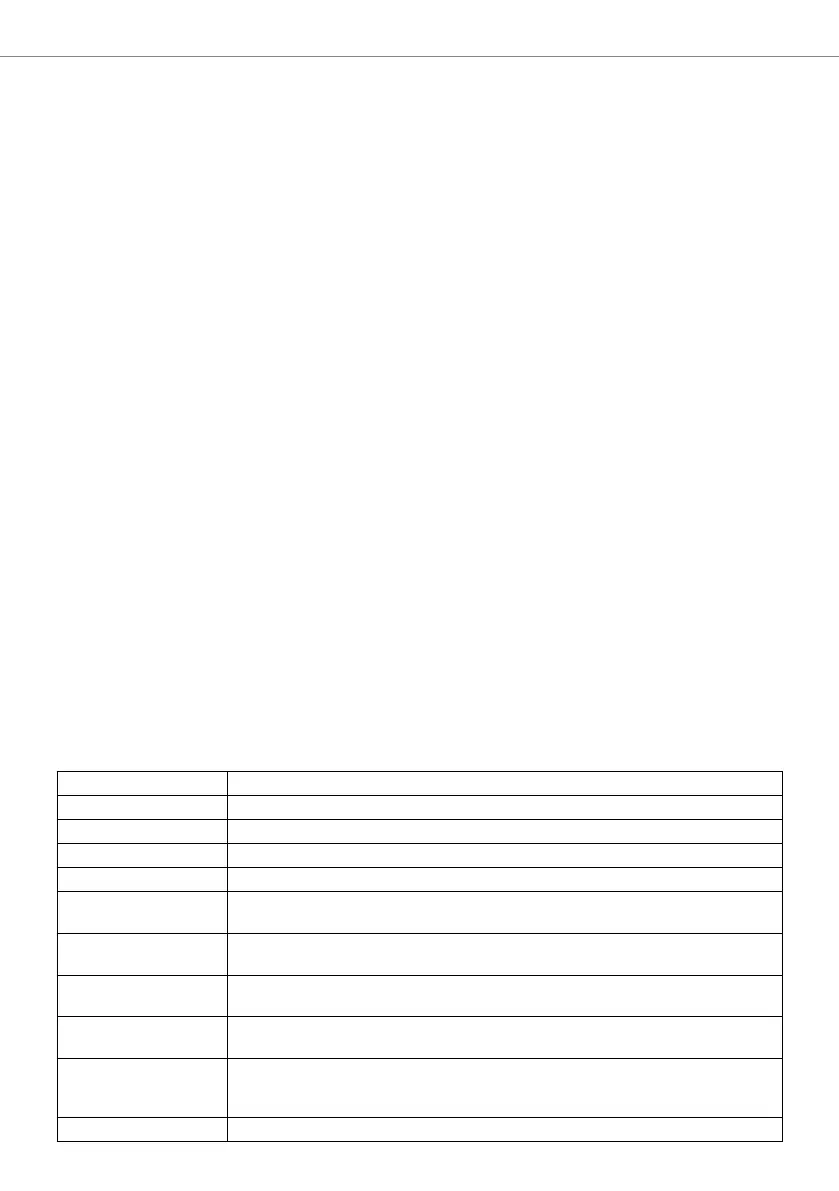
EN 980:’08 Symbols for use in the labeling of medical devices
EN 1041:’08 Information supplied by the manufacturer of medical devices
EN 1639:’09 Dentistry - Medical devices for dentistry - Instruments
EN ISO 10650-1:’05 Dentistry - Powered polymerization activators - Part 1: Quartz tungsten halogen lamps
EN ISO 10650-2:’07 Dentistry - Powered polymerization activators - Part 2: Light-emitting diode (LED) lamps
EN ISO 10993-1:’09 Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk
management process
EN ISO 17664:’04 Sterilization of medical devices - Information to be provided by the manufacturer for the
processing of resterilizable medical devices
EN 60601-1:’05 Medical electrical equipment - Part 1: General requirements for basic safety and essential
performance
EN 60601-1-2:’07 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential
performance. Collateral standard. Electromagnetic compatibility. Requirements and tests
IEC 60601-2-57:’11 Medical electrical equipment - Part 2-57: Particular requirements for the basic safety and
essential performance of non-laser light source equipment intended for therapeutic,
diagnostic, monitoring and cosmetic/aesthetic use
EN 62471:’08 Photobiological safety of lamps and lamp systems
 Loading...
Loading...