Cleaning and disinfection
Vscan Air – User Manual 6-7
Direction GP092020-1EN Rev 18
Cleaning and disinfection
Adequate cleaning and disinfection between patient cases are
necessary to prevent disease transmission. All probes must be
thoroughly cleaned prior to disinfection. The level of disinfection
required is based on patient contact. Use the following guidance
to determine the appropriate level of disinfection based on
system use.
Vscan Air is not intended for intra-operative use, it is also not
intended for intra-cavitary use. It may be used during
interventional procedures such as biopsy which based on
proximity of the probe to the needle injection site it could get
contaminated with blood or bodily fluids during use.
Chemicals Used for Efficacy Validation
The table below lists the products and intended use (cleaning,
Intermediate-level disinfection, high-level disinfection) that were
validated with the Vscan Air CL probe.
Use Method
Contact with non-intact skin Cleaning followed by High-Level Disinfection
Contact with intact skin Cleaning followed by Intermediate-Level Disinfection
Cleaning followed by Low-Level Disinfection
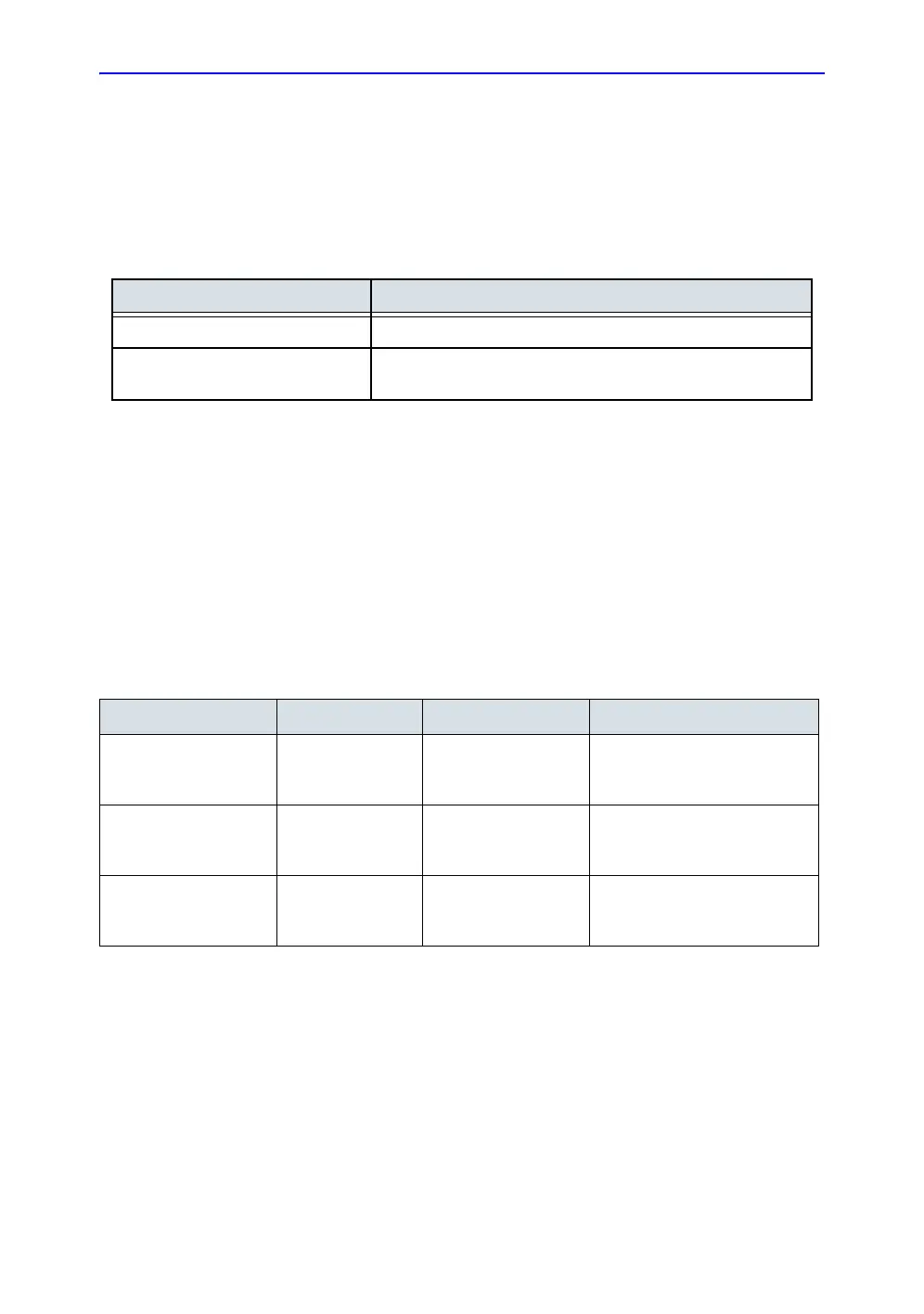
Table 6-1: Chemicals used for Efficacy Validation with Vscan Air CL
Product Type Trade Name Manufacturer Active Ingredients
Cleaning (Wipe) Sani-Cloth Prime
Germicidal
Disposable Wipe
PDI Isopropanol, Ethyl Alcohol
(Ethanol), Didecyl dimethyl
ammonium chloride
Intermediate-level
Disinfection (wipe)
Sani-Cloth Prime
Germicidal
Disposable Wipe
PDI Isopropanol, Ethyl Alcohol
(Ethanol), Didecyl dimethyl
ammonium chloride
High-Level Disinfection
(Solution)
Cidex OPA
Solution
Advanced
Sterilization Products
(J&J)
Ortho-Phthalaldehyde
 Loading...
Loading...