187692(10) - EN - P280 Mattress Instructions for Use Page 3
Destination
Specifications
Safety and Usage Tips
Intended Use
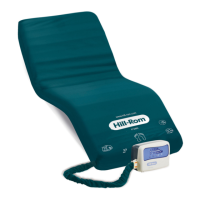
The P280, MRS (Mattress Replacement System) model with foam base or
Overlay model and P280 Air, is a therapeutic mattress designed by Hill-Rom.Its
benefits are an efficient automatic redistribution of pressure, especially in the
sacrum and heel zones, as part of a protocol for prevention and appropriate
treatment of pressure ulcers.
Indications
This device helps to prevent and treat pressure ulcers in low to high risk adult
patients, within the recommended patient weight limits of 30 to 140 kg,
in order to
achieve validated clinical performance in all the typical head of bed positions.
It can be used as a mattress in the following environments, as defined in the
standard IEC 60601-2-52:
• Application Environment 2 (short-term care in hospitals or other medical
establishments);
• Application Environment 3 (long-term care in medical establishments);
• Application Environment 5 (outpatient or ambulatory care).
This device is not designed to come into direct contact with damaged skin
and
must
to be used with a sheet between the patient’s skin and the surface of the
mattress.
In accordance with the NPUAP/EPUAP directives
1
, Hill-Rom recommends that
the condition of each patient be regularly checked. For patients with special
needs, Hill-Rom recommends the use of the more suitable I-mmersion
™
Therapy
system. Caregivers are responsible for taking this decision, in accordance with
modern care practices.
Contraindications
This device must not be used with patients:
• Suffering from an unstable fracture of the spine; for any other unstable
fractures, a medical examination is necessary to decide whether the use of
the device is appropriate.
• With atypical anatomies.
• Suffering from cervical or trans-bone traction.
1. NPUAP / EPUAP - Prevention and treatment of Pressure Ulcers - Quick Reference Guide, 2014
 Loading...
Loading...