6 7
User Guide User GuideHYDRAFACIAL MD
™
Elite
™
HYDRAFACIAL MD
™
Elite
™
EN EN
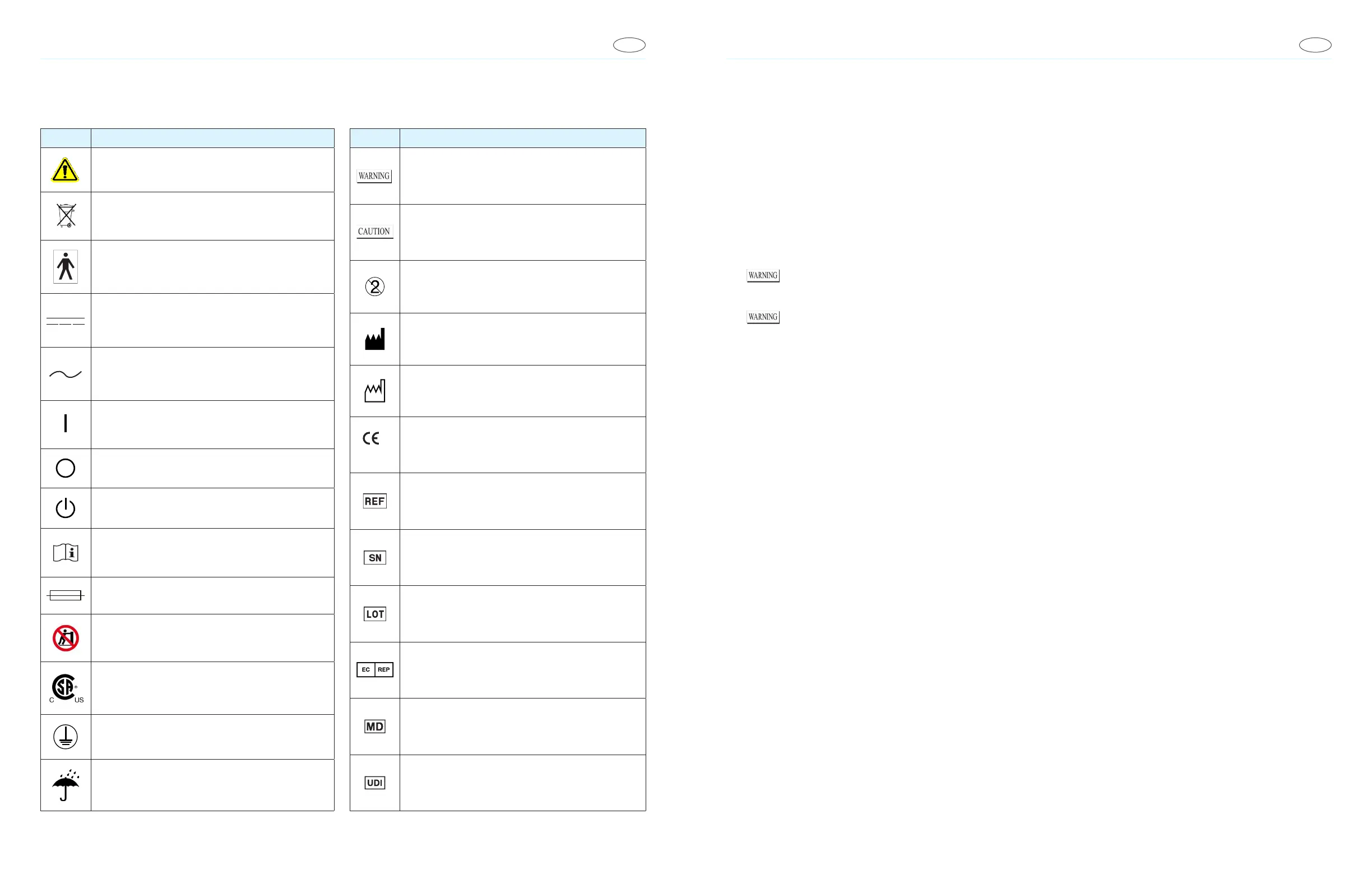
Product Marking Symbols
Symbol Description
Attention: Refer to accompanying documentation.
Separate collection for electronic.
Type BF Applied Part. Debrillation proof.
Direct Current
Alternating Current
ON (Power)
OFF (Power)
STANDBY (Power)
Consult Instructions For Use
Fuse
Pushing Prohibited
Certied by CSA Group
Connect to supply mains with protective earth (ground)
Keep Dry
Caution and Marking Symbols
Symbol Description
Calls attention to a procedure, practice, or condition that
could possibly cause bodily injury or death.
Calls attention to a procedure, practice, or condition that
could possibly cause damage to equipment or permanent loss
of data.
Do Not Re-use
Manufactured By
Date of Manufacture
2460
Conformité Européenne
Catalogue Number
Serial Number
Lot Number
European Representative
Medical Device
Unique Device Identier
Safety Information
Safety Guidelines
1. Ensure that all operators of the HydraFacial MD
™
Elite
™
System are trained and licensed as required by local/national
regulations. Do not operate the unit before being trained. For any questions regarding training, contact Edge Systems LLC or your
local distributor.
2. Be sure to read the User Guide thoroughly before setting up the System. If you experience mechanical and/or electrical difculties
with your unit, contact your local distributor.
3. Always do a client consultation to determine if the client is a candidate for the procedure. Follow contraindications and warnings
as pre-determination for procedure.
4. Always use clean, unused tips for each procedure. Keep tips in original packaging until ready to use.
Reusing tips could result in skin infection.
5. Do not use contaminated skin solutions.
Reusing contaminated skin solution could result in skin infection, and will void all warranties. Partially used skin solution
bottles must be capped and stored in accordance with instructions on product label.It is recommended not to use skin solutions
that exceed the "Best Used By" date. If you observe any abnormality with your products, please contact Edge Systems LLC or your
local distributor.
6. Removing contact lenses prior to procedure is recommended.
7. The client should have a minimum sunscreen SPF 30 applied after the procedure and should use sunscreen on an ongoing basis.
8. Each client’s skin conditions and sensitivity are different. Always begin treatment conservatively. Do a sensitivity test on the neck
by the earlobe rst, and increase or decrease the vacuum level as appropriate. Lower vacuum level is recommended for thin,
fragile skin. Skin conditions requiring more aggressive vacuum level are at operator’s discretion. Follow recommended protocols
and cautiously consider skin types.
9. Empty the waste canister after each service according to your waste handling protocol and local/national regulations. Follow
the System cleaning instructions in this guide to clean your System and handpiece.
10. Keep the System in accordance to recommended environmental conditions. Liquid should only be suctioned through
the HydraFacial handpiece.
11. Always use solution when performing abrasion. Performing the treatment without skin solutions could result in discomfort to
the client.
12. The System is not spill proof. Avoid spillage of any liquid on the System.
13. The System is not intended to be used in conjunction with any other System, medicine or technology and should only be used
in accordance with these Instructions for Use.
14. Use of non-approved skin solutions will void the warranty and may clog the System.
15. Do not let the waste canister overll. If this happens, a built-in oat device will occlude the vacuum opening and skin solution
ow through the handpiece will cease.
Note: Always remove the waste canister before transporting the unit.
16. Do not attempt to lift the unit alone or move over uneven or damaged ooring.
 Loading...
Loading...