Instructions for use PROPHYflex 3 - 2018 for Sirona connection - 1.006.9920, - 1.006.9927
7 Reprocessing steps in accordance with ISO 17664 | 7.1 Preparation at the site of use
7 Reprocessing steps in accordance with ISO 17664
7.1 Preparation at the site of use
WARNING
Hazard from incorrectly reprocessed products.
Contaminated products are associated with an infection hazard.
▶ Take suitable personal protective measures.
▶ Immediately remove any residual blood.
▶ Reprocess the medical device as soon as possible after treatment.
▶ Unscrew the powder container from the handpiece by turning it in anticlockwise
direction.
▶ Empty the powder container before reprocessing the medical device.
▶ All residual powder has to be removed, especially from the cannula, the tubes and
the P-nozzle.
▶ The medical device must be dry when transported to reprocessing.
▶ Do not place in solutions or similar substance.
7.2 Cleaning
NOTICE
Never reprocess this medical device in an ultrasonic device.
Malfunction and material damage.
▶ Clean manually or in a washer disinfector only.
7.2.1 Manual cleaning of the exterior
Accessories required:
▪ Tap water 30
o
C ± 5
o
C (86
o
F ± 10
o
F)
▪ Brush, e.g. medium-hard toothbrush
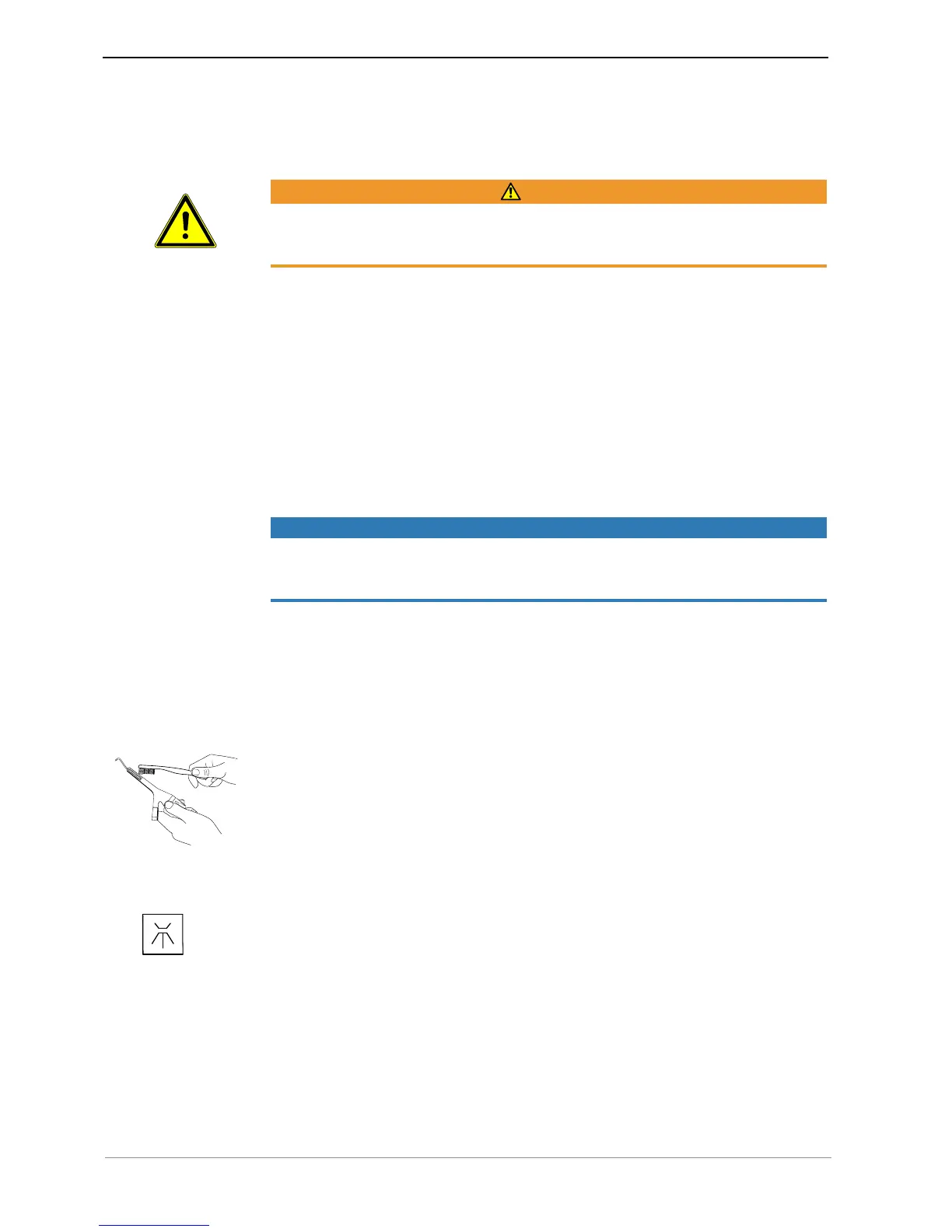
▶ Brush off under flowing tap water.
7.2.2 Automated external cleaning
KaVo recommends washer disinfectors in compliance with EN ISO 15883-1 that are
operated with alkaline cleaning agents.
The validations were conducted with the VARIO-TD program, the cleaning agent
neodisher® MediClean and the neutralisation agent neodisher® Z.
▶ For program settings as well as cleansers and disinfectants to be used, please
refer to the Instructions for Use of the thermodisinfector.
19 / 26

 Loading...
Loading...