13.1 Declaration of conformity
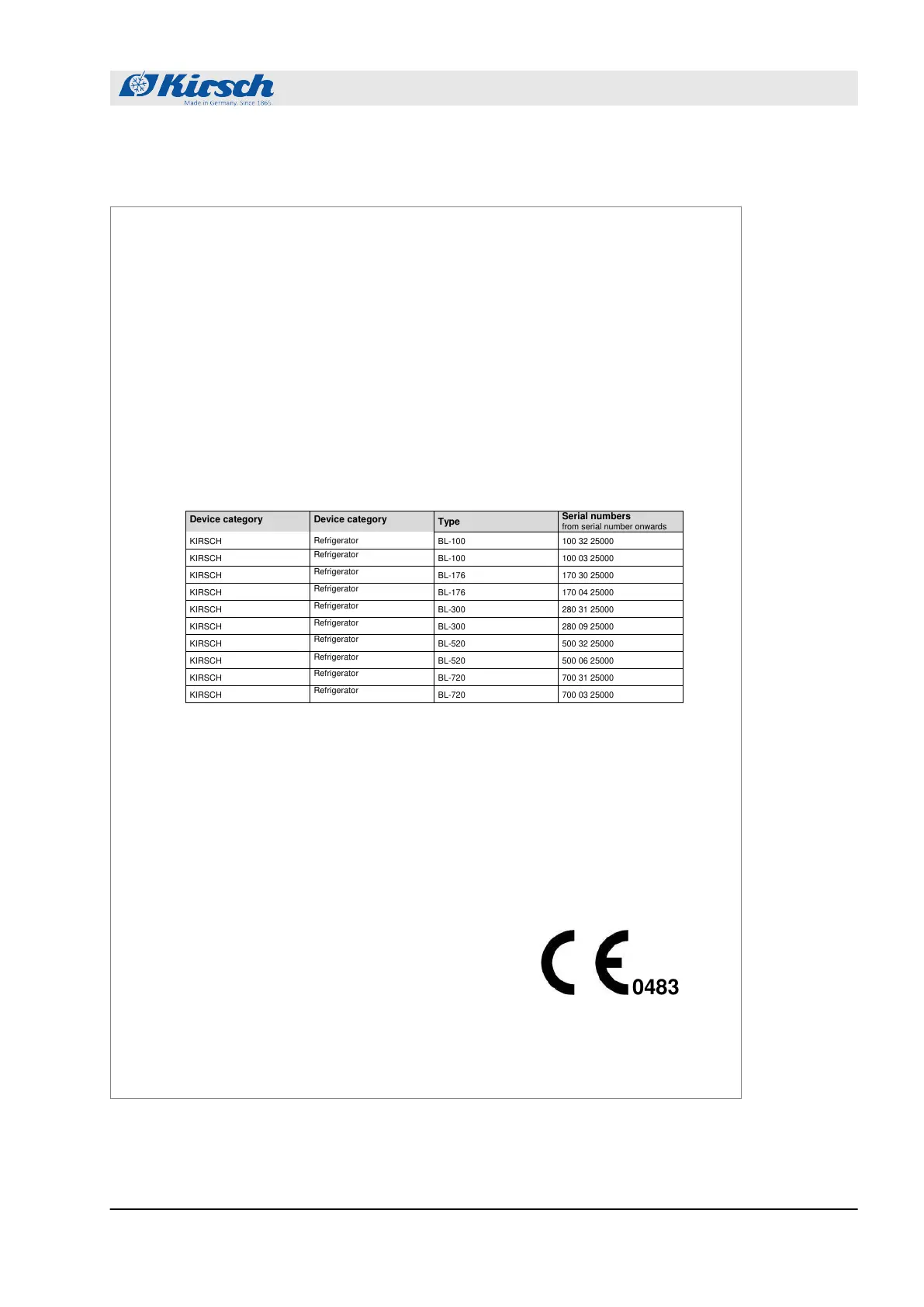
Serial numbers
from serial number onwards
Appendix
Declaration of conformity
18.12.2018 Refrigerator/freezer BL series 73
Directives
DIN EN ISO 9001:2015
DIN EN ISO 13485:2016
DIN EN ISO 14971:2012
DIN 58371:2010-09
Harmonised standards
EN 55014-1:2006/A2:2011
EN 55014-2:1997/A2:2008
EN 60335-1:2012
EN 60335-2-89:2010
EN 60601-1-2:2015 chapter 7, 8
IEC 60601-1-2:2014 chapter 7, 8
EN/IEC 64000-3-2:2014
EN/IEC 61000-3-3:2013
EC Declaration of Conformity
We,
Philipp Kirsch GmbH
Im Lossenfeld 14
77731 Willstätt-Sand
Germany
hereby declare on our sole authority that the devices of type BL described below, to which the declaration refers,
comply with the applicable requirements of Directive 93/42 EEC for medical devices.
The devices belong to Class IIa according to Appendix IX of the Directive above. The named authority, mdc medical
device certification GmbH, Kriegerstraße 6, 70191 Stuttgart, s included in the conformity procedure according to
Appendix II of Directive 93/42/EEC.
Registration-Nr.: D1423300009
We furthermore declare that the devices described below comply with the applicable requirements of the RoHS
Directive 2011/65/EC and the protection requirements of the standards below at the time that the device was placed
on the market.
 Loading...
Loading...




