Do you have a question about the Mindray BeneHeart S1 Fully Automatic and is the answer not in the manual?
Provides essential safety information, including dangers, warnings, cautions, and notes.
Explains the meaning of various symbols used on the equipment and in the manual.
Provides a general overview of the BeneHeart C & S series defibrillators and their models.
Defines the intended application and target patient population for the defibrillator.
Lists the parts of the equipment that are applied to the patient during use.
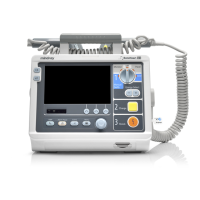
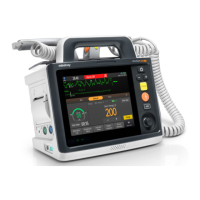
Details the components and layout of the main unit, including top, bottom, and back views.
Outlines crucial safety precautions and requirements before installing and using the equipment.
Guides through the process of unpacking, checking, and installing the equipment and its accessories.
Provides instructions on how to safely power on the defibrillator unit.
Explains how to change the voice prompt language for the equipment.
Details the procedure for safely powering off the defibrillator unit.
Details critical safety precautions during operation, including dangers and warnings.
Explains the elements and indicators displayed on the equipment's screen.
Outlines the general steps for responding to a rescue situation with the defibrillator.
Details how to perform Cardiopulmonary Resuscitation (CPR) as guided by the equipment.
Provides steps for preparing the equipment for a subsequent rescue operation.
Details the types of data stored and how to manage them using the equipment.
Explains how the equipment automatically generates patient files for recording clinical data.
Guides on managing equipment configurations using the AED Tool software.
Provides an overview of the AED ALERT Device Management system features and usage.
Details the procedure for accessing the AED ALERT system via the internet.
Introduces the disposable battery used by the equipment.
Provides essential safety guidelines for handling and using the equipment's battery.
Explains how to interpret on-screen indicators and voice prompts for battery status.
Provides step-by-step instructions for safely replacing the equipment's battery.
Offers guidance on the proper storage of batteries to maintain their performance.
Provides instructions for the proper disposal and recycling of used batteries.
Outlines general rules and precautions for keeping the equipment clean and free of damage.
Provides specific instructions and recommended agents for cleaning the equipment.
Explains the procedure for disinfecting the equipment as per facility servicing schedules.
States that sterilization is not recommended unless specifically indicated.
Introduces the importance of periodic testing and maintenance for equipment functionality.
Details critical safety precautions and warnings related to equipment maintenance.
Outlines recommended tests and maintenance procedures like user and auto tests.
Provides guidance on the proper disposal of the equipment and its accessories at end-of-life.
Lists and describes therapy accessories compatible with the defibrillator equipment.
Lists miscellaneous accessories, such as disposable batteries.
Details the safety classifications and standards the equipment complies with.
Specifies the operating and storage conditions for temperature, humidity, and barometric pressure.
Lists the physical dimensions and weight of the main unit of the equipment.
Details the specifications of the equipment's display screen, including type, size, and resolution.
Describes the audio output capabilities and specifications of the equipment.
Details the specifications for various interface connectors like USB and network.
Provides technical details about the equipment's battery, including capacity and operating time.
Specifies the data storage capabilities of the equipment for waveforms, events, and voice.
Details the wireless communication standards and frequencies used by the equipment.
Lists the technical specifications related to the defibrillation function, energy, and analysis.
Specifies the ECG input, gain, sweep speed, and recovery time for the equipment.
Details the specifications of different electrode pads, including area, adhesive area, and shelf life.
Describes the methods and databases used for rhythm recognition and annotation.
Presents test results on the performance of the rhythm analysis algorithm against standards.
Details the Electromagnetic Compatibility requirements and guidance for the equipment.
Provides information on radio regulatory compliance and electromagnetic immunity.
Lists the default settings for general system parameters like date, time, and language.
Details the default setup options for the Automatic External Defibrillator (AED) functions.
Outlines the default settings for Cardiopulmonary Resuscitation (CPR) related functions.
Specifies the default settings for auto test intervals and transmission.
Details the default settings for configuring Wi-Fi and cellular network connections.
Lists the default settings for AED ALERT system related features like reminders and data upload.
Lists voice prompts that may occur during a rescue and their corresponding descriptions.
Lists common units of measurement used in the manual and their full names.
Defines common symbols used in the manual and their meanings.
Provides a list of abbreviations and acronyms used in the manual and their full forms.
Provides a checklist for daily inspection of the equipment's status indicator.
Includes a section for recording the expiration date of electrode pads.
Information for tracking the product to provide high quality service.
Formal declaration of the product's conformity with relevant EU directives and standards.
| Display | LCD |
|---|---|
| Waveform | Biphasic |
| Voice Prompts | Yes |
| CPR Guidance | Yes |
| CPR Feedback | Yes |
| ECG Analysis | Yes |
| ECG Monitoring | Yes |
| Self-Test | Yes |
| Data Recording | Yes |
| Data Storage | Yes |
| Data Transfer | USB |
| Connectivity | USB |
| Operating Temperature | 0°C to 50°C |
| Storage Temperature | -20°C to 60°C |
| IP Rating | IP55 |
| Altitude | Up to 4000m |










 Loading...
Loading...