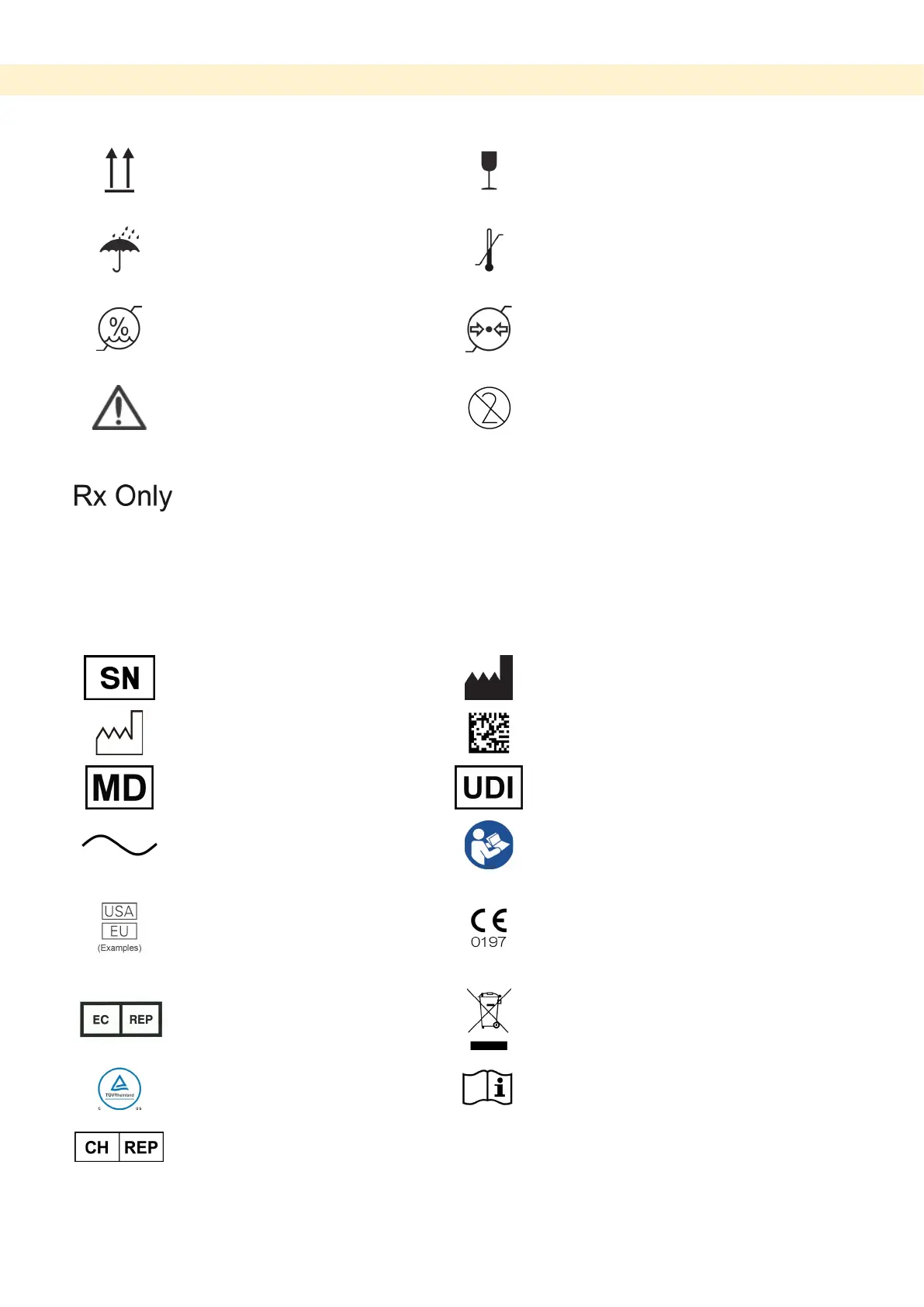
Package
This way up
Fragile
Keep away from rain
Temperature limitation
Humidity limitation
Atmospheric pressure limitation
Attention, consult accompany
documents
Do not reuse
Prescription Device
CAUTION: Federal law restricts this
device to sale by or on the order of a
dentist and a licensed healthcare
practitioner.
Rating Label, X-ray Tube Head Assembly Label, and Instructions for Use
Serial number
Manufacturer
Date of manufacture
GS1 DataMatrix
Medical device
Unique device identifier
Alternating current
Refer to instructions for use
Country or region
(Country Names: Conforming to the ISO 3166-1
alpha-3 codes)
Description noted next to the code is an indication
that conforms to the regulations valid only for the
relevant country or region.
CE(0197) marking (Valid only for EU)
Conforms with the European Directive,
93/42/EEC.
CE marking (Valid only for EU)
Conforms with the European Directive,
2011/65/EU.
EU authorized representative under the
European Directive 93/42/EEC
(Valid only for EU)
Marking of electrical equipment in
accordance with the European Directive
2012/19/EU (WEEE)
(Valid only for EU)
cTUVus certification mark
(Valid only for U.S.A. and Canada)
Consult instructions for use
Authorized representative in
Switzerland
 Loading...
Loading...