9
5 What Are the Risks of Treatment with Optune
®
?
The table below shows the occurrence of certain events when Optune was used correctly and incorrectly in the clinical
study in patients whose tumor reappeared after cancer drugs.
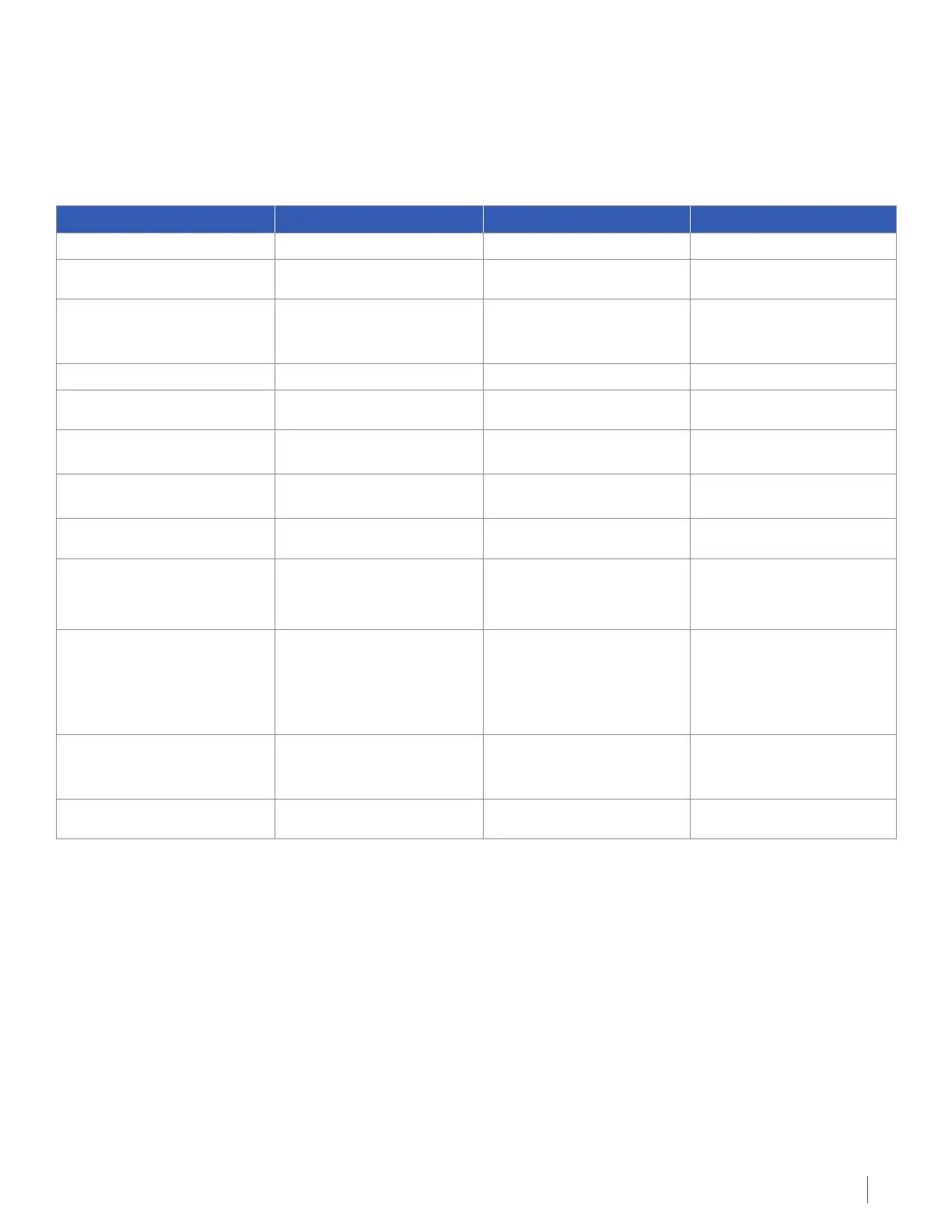
Occurrence of Certain Problems with Correct and Incorrect Use of Optune
Event Likelihood of Event Outcome/Harm Likelihood of Outcome
Correct use
Skin reaction 18 out of 116 subjects (16%) Mild scalp redness (rash) 17 out of 18 subjects (95%)
Skin reaction 18 out of 116 subjects (16%) Moderate scalp redness
(rash with little sores and
blisters)
6 out of 18 subjects (33%)
Incorrect use
Skin reaction 1 out of 116 subjects (1%) Open sore on scalp 1 out of 1 subjects (100%)
Use in a patient with a
pacemaker
1 out of 121 subjects (1%) Heart problems 0 out of 1 subject (0%)
Use in patients 21 years or
younger
0 out of 120 subjects (0%) Unknown Unknown
Use in pregnant women 0 out of 120 subjects (0%) Unknown Unknown
Use in patients with
implanted electronic devices
or bullet fragments
0 out of 120 subjects (0%) Unknown Unknown
Known allergic reaction to
electrode gels
0 out of 120 subjects (0%) Increased redness and
itching, (rarely may even
lead to severe allergic
reactions such as shock
and breathing failure)
Unknown
Opening the device for
service by untrained
personnel
0 out of 120 subjects (0%) Damage to the device and
risk of electric shock
Unknown
Incorrect uses not predicted Unknown Unknown Unknown
What Are the Risks of Treatment with Optune?
 Loading...
Loading...