8
5 What Are the Risks of Treatment with Optune
®
?
Skin irritation is often seen under the transducer arrays when using Optune. This will look like a red rash, small sores or
blisters on your scalp. In general, this will not cause skin damage that cannot be fixed. The irritation can be treated with
steroid cream or by moving the transducer arrays. If you do not use steroid cream, the skin irritation could become
more serious. This may lead to open sores, infections, pain and blisters. If this happens, stop using the steroid cream and
contact your doctor.
In the clinical study of Optune in GBM that reappeared after chemotherapy, headaches, weakness, convulsions and
thinking changes were seen. In the device group, 18 out of 116 patients had headaches, 10 out of 116 patients had
weakness, 11 out of 116 patients had convulsions and 6 out of 116 patients had thinking changes. These events are
also seen in patients with recurrent GBM who do not use Optune. However, there was a higher rate of these problems
overall in Optune patients (43.1%) compared to patients on cancer drugs (36.3%). Only skin redness and open sores are
related to Optune treatment itself.
By using Optune instead of cancer drugs, patients would avoid many of the side eects due to cancer drugs. These
include infections, nausea, vomiting, loss of appetite, and tiredness. Three times as many patients who used cancer
drugs had these side eects compared to patients who used Optune.
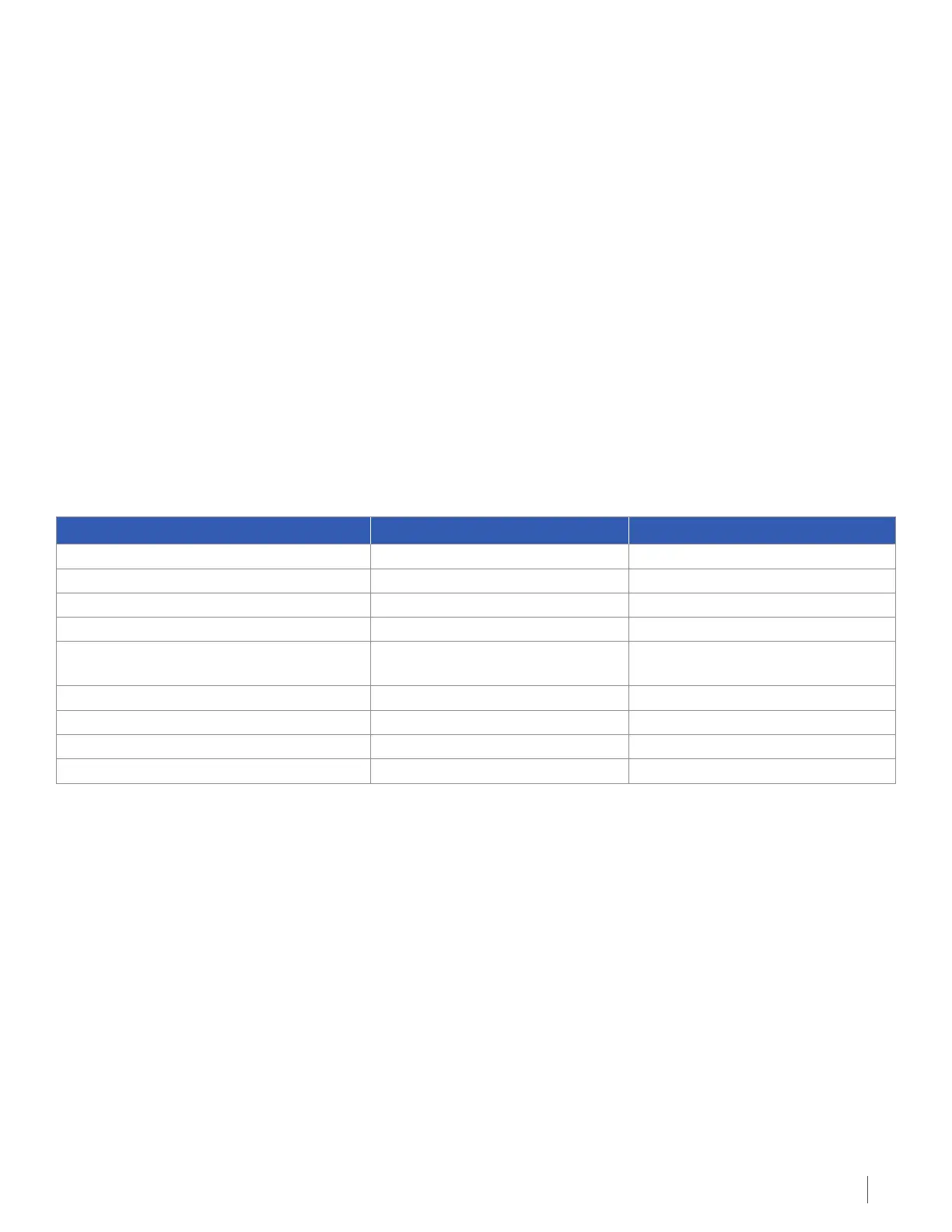
The table below shows the occurrence of medical problems in patients using Optune after cancer drugs compared to
patients on cancer drugs.
Occurrence of Medical Problems in Patients Using Optune Compared to Patients on Cancer Drugs
Medical Problem Optune Cancer Drugs
Lower white and red blood cell counts 5 out of 116 subjects (4%) 17 out of 91 subjects (19%)
Vomiting, nausea and diarrhea 9 out of 116 subjects (8%) 27 out of 91 subjects (30%)
General disorders 15 out of 116 subjects (13%) 14 out of 91 subjects (15%)
Infections 5 out of 116 subjects (4%) 11 out of 91 subjects (12%)
Rash under device transducer arrays and
other injuries
21 out of 116 subjects (18%) 1 out of 91 subjects (1%)
Nutrition disorders 9 out of 116 subjects (8%) 12 out of 91 subjects (13%)
Brain disorders 50 out of 116 subjects (43%) 33 out of 91 subjects (36%)
Behavioral disorders 12 out of 116 subjects (10%) 7 out of 91 subjects (8%)
Breathing disorders 7 out of 116 subjects (6%) 10 out of 91 subjects (11%)
What Are the Risks of Treatment with Optune?\\DC - 099263/000001 - 12620961 v1
 Loading...
Loading...