18
3
GENERAL INFORMATION: BATTERIES
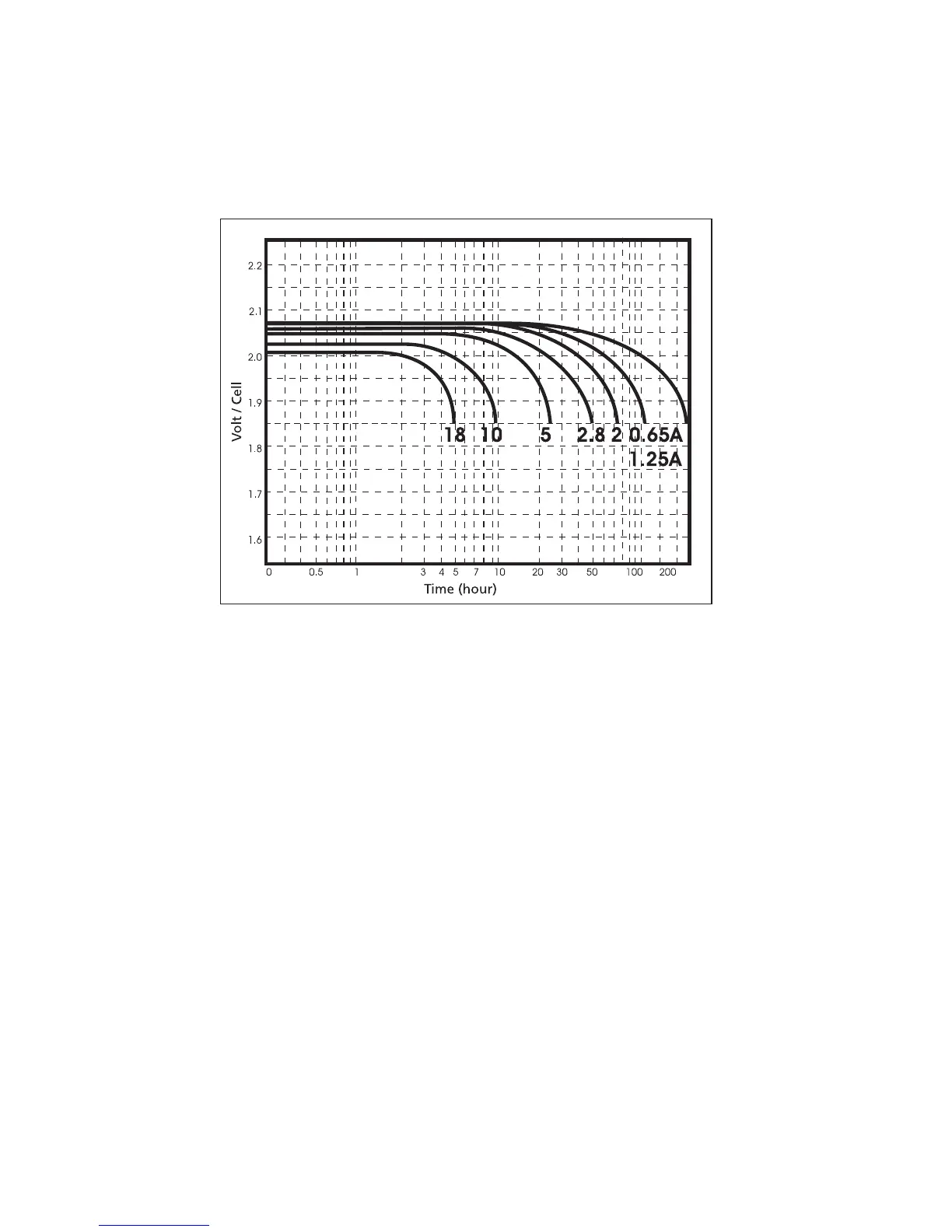
Fig 3.3: Discharge characteristic curves
Figure 3.3 shows the discharge prole of a typical battery type at several constant
current rates. The typical end-of-discharge voltage at these discharge rates can
also be noticed where the voltage starts to drop steeply. Moreover, the end-of-
discharge voltage varies between 1.75-1.9 V, depending on the battery type and the
discharge current. Higher service capacity is obtained at the lower discharge rates.
At higher discharge rates, the electrolyte in the pore structure of the plate becomes
depleted, and it cannot diffuse rapidly enough to maintain the cell voltage. However,
intermittent discharge, which allows time for electrolyte diffusion will improve the
performance under high discharge rates
Gassing
With 2.3 V – 2.4 V, namely the so-called Gassing Voltage, gas is developed at the
electrodes in the battery, by which the water is decomposed into hydrogen (H
2
)
and oxygen(O
2
). Both gases mix together in the battery providing detonating gas
(explosive!) and escape through ventilation opening in the vent plug. With the
gassing, the battery also loses water, which must be relled according to maintenance
within regular intervals. The gas is the unwelcome secondary reaction of the chemical
conversion during charging because current is consumed for the electrolysis and,
therefore, the storage efciency of the battery is made worse unnecessarily.
 Loading...
Loading...