35
Note: The results provided are for n=3 devices tested in triplicate for each
drug.
The results provided for the Aerogen
®
Solo nebulizer are reflective of the
Aerogen
®
Pro nebulizer, as the fundamental scientific technology in both
nebulizers is the same.
Table 12. Performance Specifications of the Aerogen Pro
Flow Rate >0.2 mL/min (Average: ≈ 0.4 mL/min)
Particle
Size
As measured with the Andersen Cascade Impactor:
• Specification Range: 1-5 μm
• Average Tested: 3.1 μm
As per EN 13544-1, with a starting dose of 2 mL:
• Aerosol Output rate: 0.24 mL/min
• Aerosol Output: 1.08 mL emitted of 2.0 mL dose
• Residual Volume: <0.1 mL for 3 mL dose
Performance may vary depending upon the type of drug and nebulizer unit used. For
additional information contact Aerogen or drug supplier.
The temperature of the medication will not rise more than 10ºC (18ºF) above ambient
temperature during normal use.
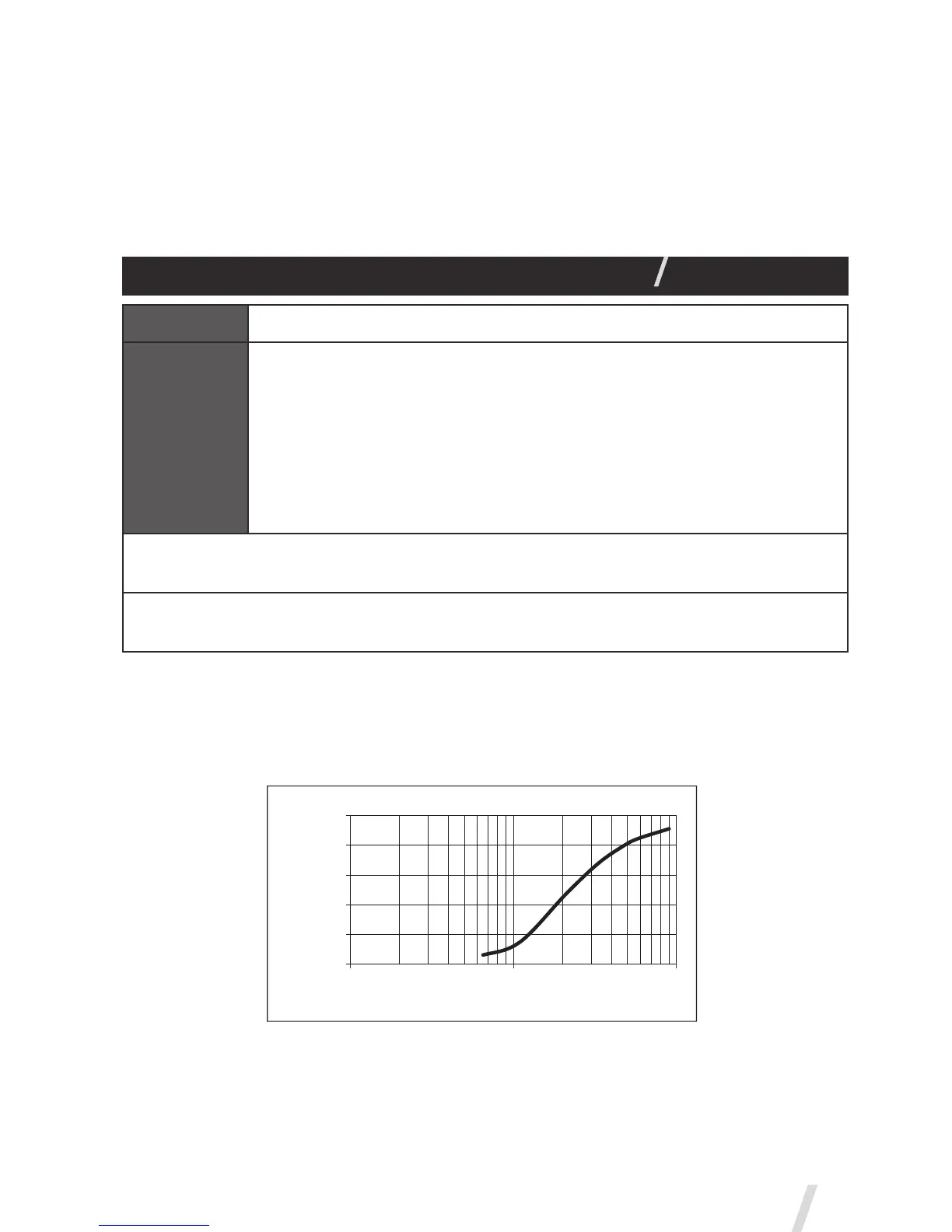
Representative particle size distribution for Albuterol as per EN 13544-1 is
shown below for the Aerogen Pro.
0%
20%
40%
60%
80%
100%
0.1 1 10
Particle Size (µm)
Cumulative Undersize %
 Loading...
Loading...