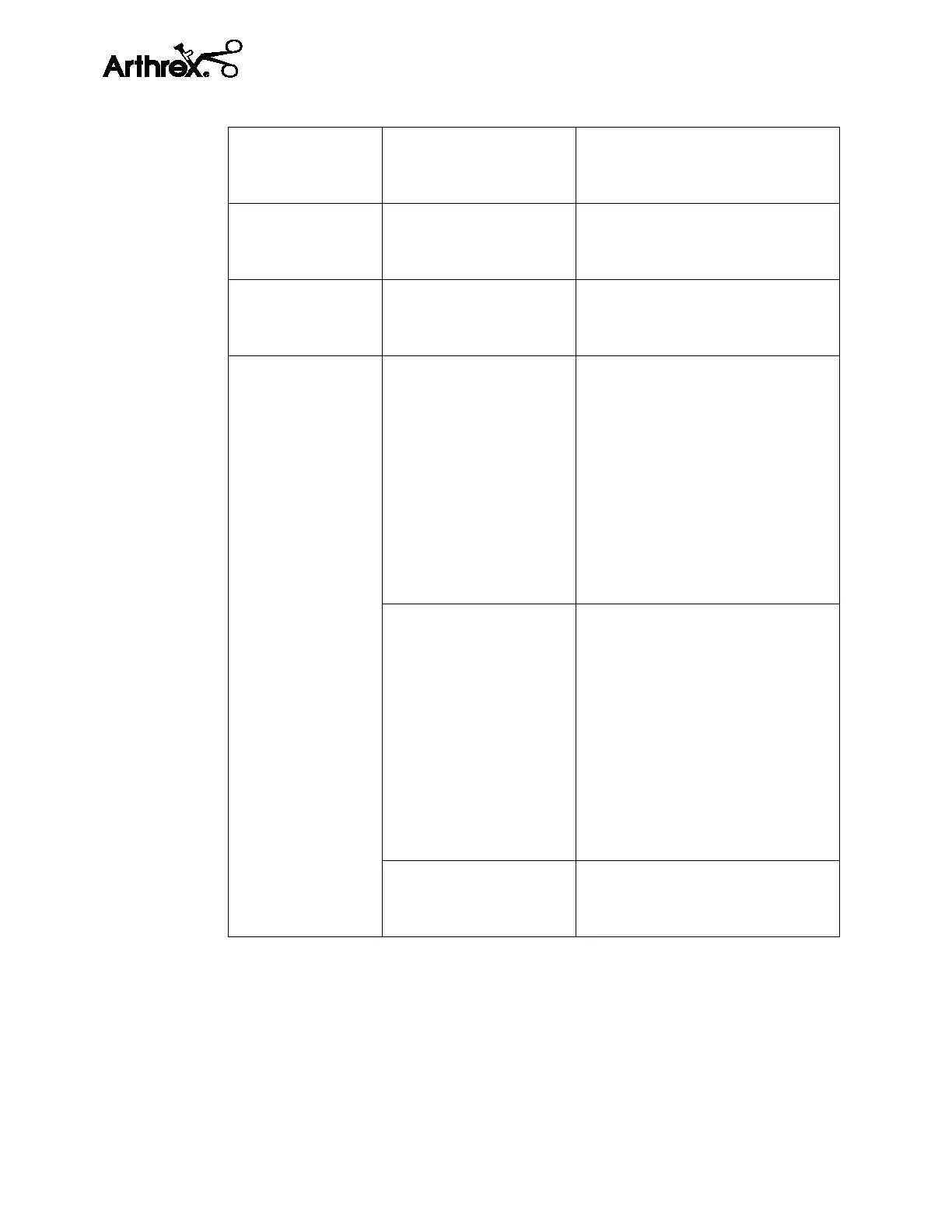
Technical Specifications
NanoScope Manual
DFU-0298-1, Revision 0 Page 19 of 38
According to the mode of
operation
Continuous
CE Classification
93/42/EEC
Annex IX
Console Class I per Rule 12
Handpiece Class IIa Rule 6
FCC Certification IEEE 811.20 FCC ID: Z64-WL18DBMOD
Radio Module
Information
FCC Information
This device complies with Part 15 of
the FCC Rules. Operation is subject to
the following two conditions: (1) this
device may not cause harmful
interference, and (2) this device
must accept any interference
received, including interference that
may cause undesired operation.
CAUTION: changes or modifications
not expressly approved by Arthrex
will void the user’s authority to
operate this equipment.
Industry Canada
Information:
Contains IC: 451I-WL18DBMOD
IC Model: WL18MODGI
This device complies with Industry
Canada license-exempt Radio
Standard Specification (RSS)
standard(s). Operation is subject to
the following two conditions; (1) this
device may not cause harmful
interference, and (2) this device
must accept any interference
received, including interference that
may cause undesired operation.
European Union
Information:
Compliant with (RED)(2014/53/EU)
For all other NanoScope Handpiece Kit and accessories refer to DFU-0266-XX for
more information.
Refer to Section 12.0 Electromagnetic Emissions for further details on EMC
Certification.

 Loading...
Loading...