58 59
Contour
®
next GEN blood glucose monitoring system
TECHNICAL INFORMATION
Intermediate measurement precision (which includes variability across
multiple days) was evaluated using control solutions at 5 glucose
levels. With each control solution, each of 3 lots of Contour next test
strips was tested once on each of 10 instruments on 10 separate days
for a total of 300 readings. The following precision results
were obtained.
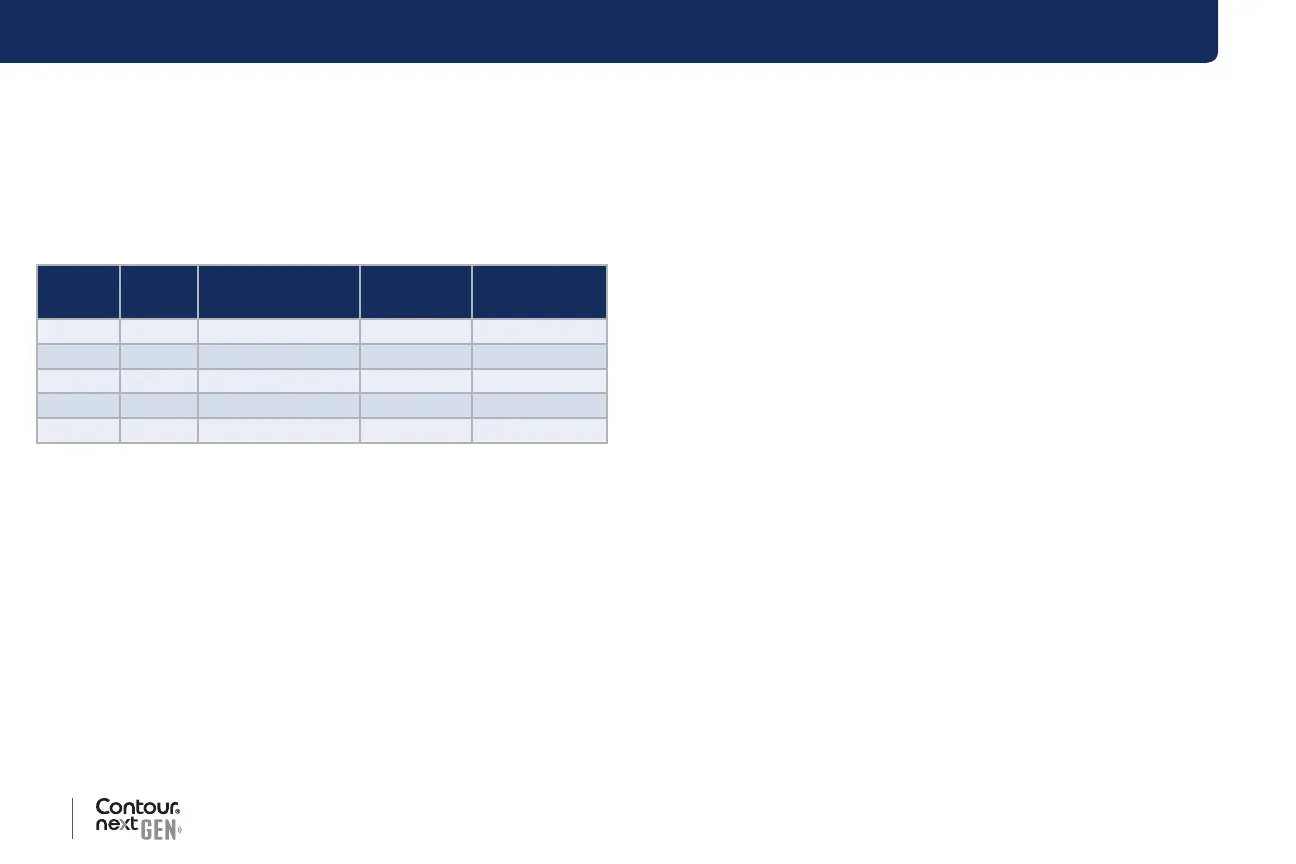
Table 2: System intermediate precision results for
Contour next GEN meter using Contour next test strips
Control
Level
Mean,
mg/dL
Pooled Standard
Deviation, mg/dL
95% CI of
SD, mg/dL
Coecient of
Variation, %
1 43.1 0.6 0.56–0.66 1.4
2 83.9 1.1 0.98–1.17 1.3
3 126.3 1.5 1.41–1.67 1.2
4 219.9 2.9 2.64–3.12 1.3
5 370.6 6.5 6.04–7.15 1.8
Federal Communications Commission (FCC)
Certied Device
This equipment has been tested and found to meet the limits for a
Class B digital device, pursuant to part 15 of the FCC Rules. These
limits are designed to provide reasonable protection against harmful
interference in a residential installation. This equipment generates,
uses, and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause harmful
interference to radio communications. However, there is no guarantee
that interference will not occur in a particular installation. If this
equipment does cause harmful interference to radio or television
reception, which can be determined by turning the equipment o and
on, the user is encouraged to try to correct the interference by one or
more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
This portable transmitter with its antenna complies with FCC/IC RF
exposure limits for general population/uncontrolled exposure.
This device complies with part 15 of the FCC Rules. Operation is
subject to the following 2 conditions: (1) This device may not cause
harmful interference, and (2) this device must accept any interference
received, including interference that may cause undesired operation.
Compliance with these guidelines means that under normal, daily
circumstances, the device should not aect the operation of other
devices. In addition, the device should operate normally in the
presence of other devices.
In the event there is interference from another device, it is recommended
that you increase the distance between the meter and that device.
You can also turn o the interfering device. In addition, you can turn o
Bluetooth wireless technology on the meter.
Changes or modications to the device not expressly approved by
Ascensia Diabetes Care could void the user’s authority to operate the
device.
If you have questions, contact Customer Service. See back cover for
contact information.
 Loading...
Loading...