60 61
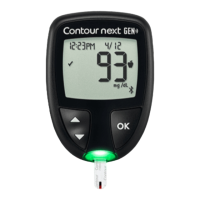
Contour
®
next GEN blood glucose monitoring system
TECHNICAL INFORMATION
Specications
Test Sample: Capillary whole blood only
Test Result: Referenced to plasma/serum glucose
Sample Volume: 0.6 μL
Measuring Range: 20 mg/dL–600 mg/dL of glucose in blood
Countdown Time: 5 seconds
Memory: Stores most recent 800 test results
Battery Type: Two 3-volt CR2032 or DL2032 coin cell batteries,
225 mAh capacity
Battery Life: Approximately 1000 tests (1 yr. average use, 3 tests per day)
Meter Operating Temperature Range: 41°F–113°F
Control Testing Temperature Range: 59°F–95°F
Meter Operating Humidity Range: 10% RH–93% RH
Test Strip Storage Conditions: 41°F–86°F, 10%–80% Relative
Humidity (RH)
Dimensions: 78.5 mm (L) x 56 mm (W) x 18 mm (H)
Weight: 53 grams
Meter Use Life: 5 years
Sound Output: 45 dB(A)–85 dB(A) at a distance of 10 cm
Radio Frequency Technology: Bluetooth Low Energy
Radio Frequency Band: 2.4 GHz–2.483 GHz
Maximum Radio Transmitter Power: 1 mW
Modulation: Gaussian Frequency Shift Keying (GFSK)
Electromagnetic Compatibility (EMC): The
Contour next GEN
meter complies with the electromagnetic requirements specied in
IEC 60601-1-2 Edition 4.0. Electromagnetic emissions are low and
unlikely to interfere with other nearby electronic equipment, nor are
emissions from nearby electronic equipment likely to interfere with the
Contour next GEN meter.
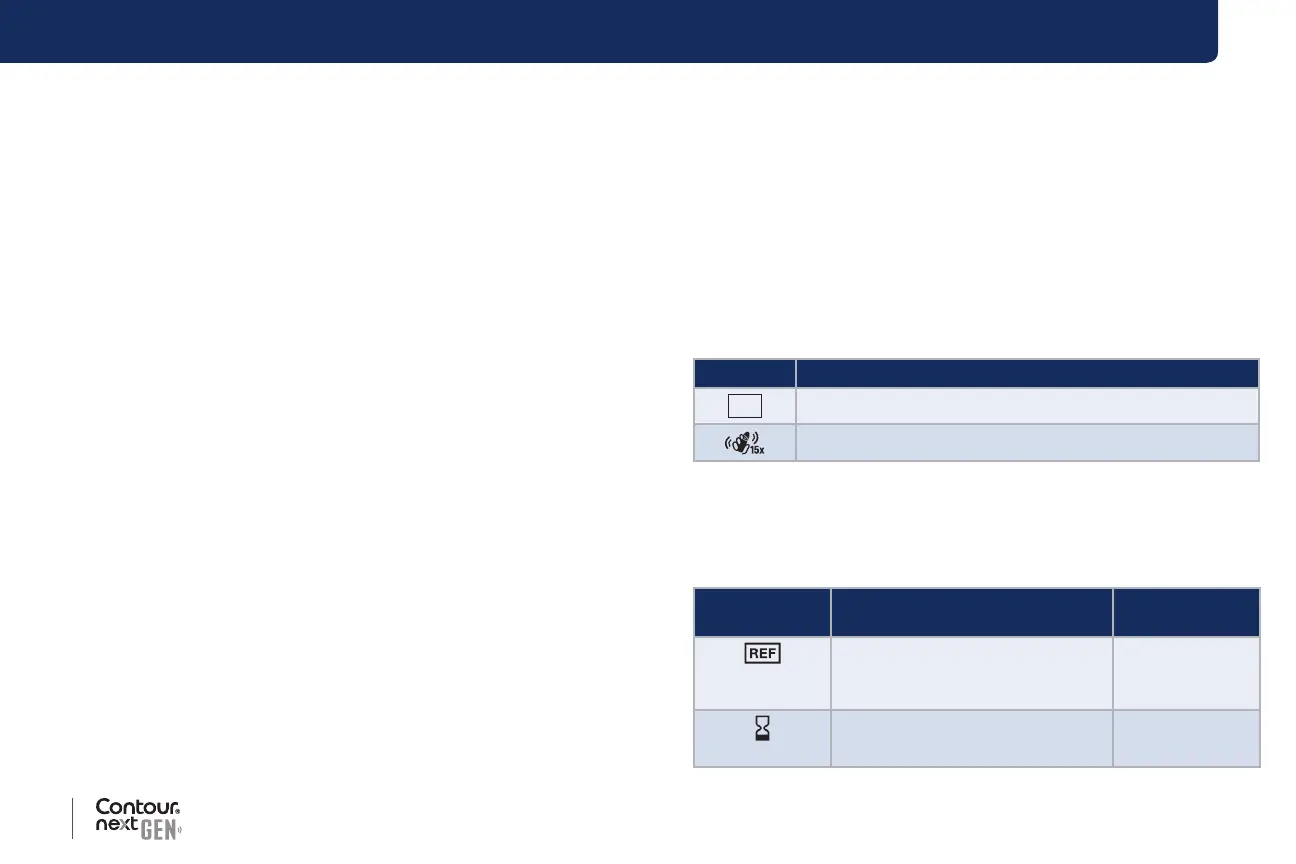
Product Labeling Symbols
The following symbols are used throughout the product labeling for
the
Contour next GEN blood glucose monitoring system (meter
packaging and labeling, and test strip and control solution packaging
and labeling).
Symbol What It Means
Discard
Date:
Control Discard Date
Shake 15 times
The following symbols from the International Organization for
Standardization (ISO) and International Electrotechnical Commission (IEC)
are used throughout the product labeling for the
Contour next GEN
blood glucose monitoring system (meter packaging and labeling, and test
strip and control solution packaging and labeling).
Symbol
Graphic/Title
Explanatory Text
Standard
Reference
6-8
Catalog or
model number
Indicates the manufacturer’s
catalog number so that the
medical device can be identied.
ISO 15223-1,
Clause 5.1.6
Use-by date
Indicates the date after which the
medical device is not to be used.
ISO 15223-1,
Clause 5.1.4
 Loading...
Loading...