Section 2 - Page 2 of 3
3121-9001-0167_EN.03.00_QT_Manual 04/2016
Intended Use
TheQuo-TestA1CSystemisintendedforthein vitro quantitative determination of
glycated hemoglobin (HbA1c) in whole blood obtained from a nger prick or venous
whole blood sample collected into EDTA tubes.
TheQuo-TestA1CSystemisindicatedinthemanagementandtreatmentofdiabetes
mellitus and for monitoring long term glycemic control in patients diagnosed with
diabetes.
TheQuo-TestA1CSystemisdesignedforprofessionaluseonly.
Quo-Test Quality Controls are intended to check the correct operation of the Quo-Test
A1CSystem.
Quo-Test System Description
TheQuo-TestA1CSystemconsistsoftheQuo-TestAnalyzer,Quo-TestA1CTest
Cartridges,Quo-TestA1CControlKitandThermalLabelPrinter(optional).
Quo-Test A1C Test Cartridges can only be run on the Quo-Test Analyzer.
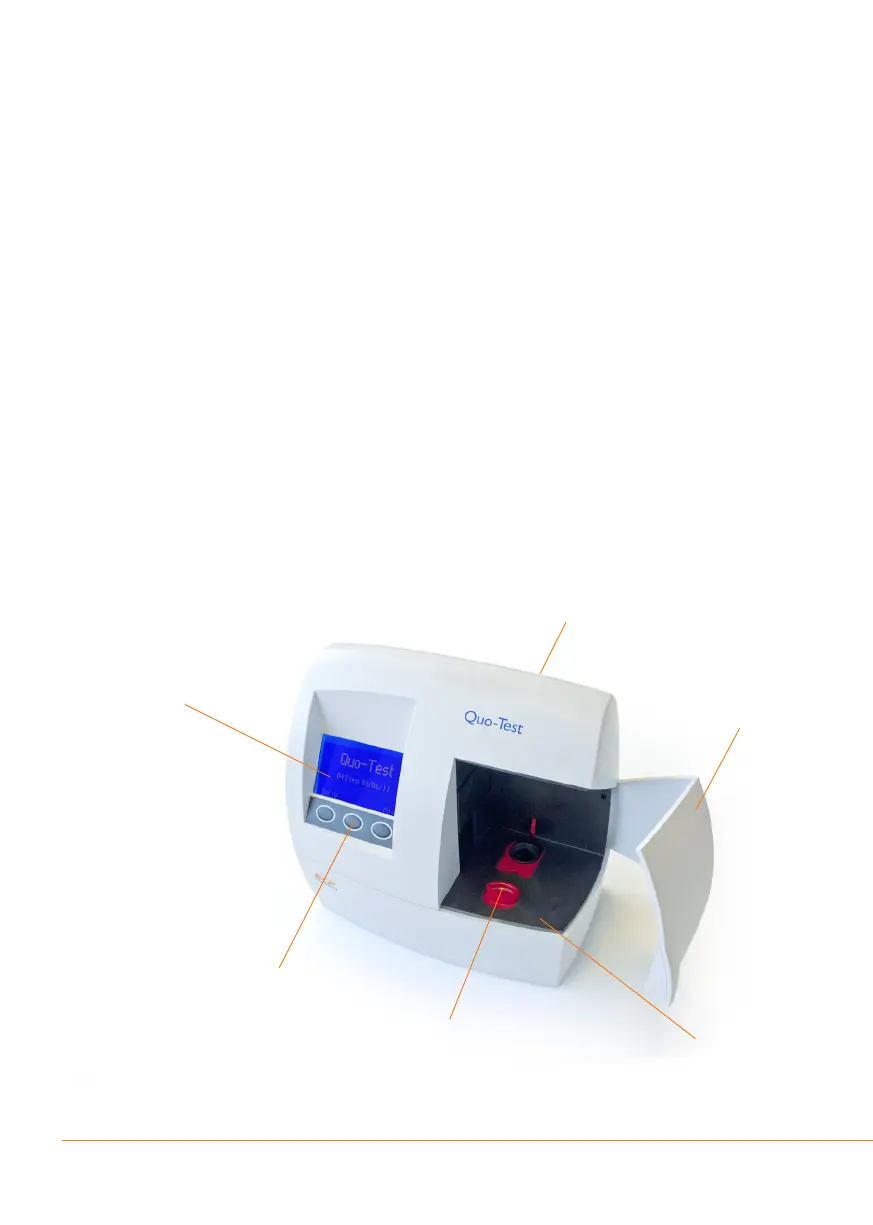
Figure 1 shows the main parts of the analyzer.
Figure 1: The Quo-Test Analyzer
Door
Rear Connection Panel
(see Figure 2 in Section 3 - Setting up the System)
Test Chamber
Key Pad
Display Screen
Slide
 Loading...
Loading...