4
Regulatory Requirements
The Clinical Laboratory Improvement Amendments (CLIA) has classifi ed tests of blood glucose as
tests that are waived. For all entities that conduct one or more tests, including waived tests
on materials derived from the human body for the purpose of providing information for the diagnosis,
prevention or treatment of any disease or impairment of, or the assessment of the health
of human beings, CLIA has stated that the entity conducting the tests shall meet certain Federal
requirements. If any entity conducts tests for the aforementioned purposes, then the entity, under
CLIA, is considered to be a laboratory and thus must register with the CLIA Program.
KEEP THESE INSTRUCTIONS
IN A SAFE PLACE
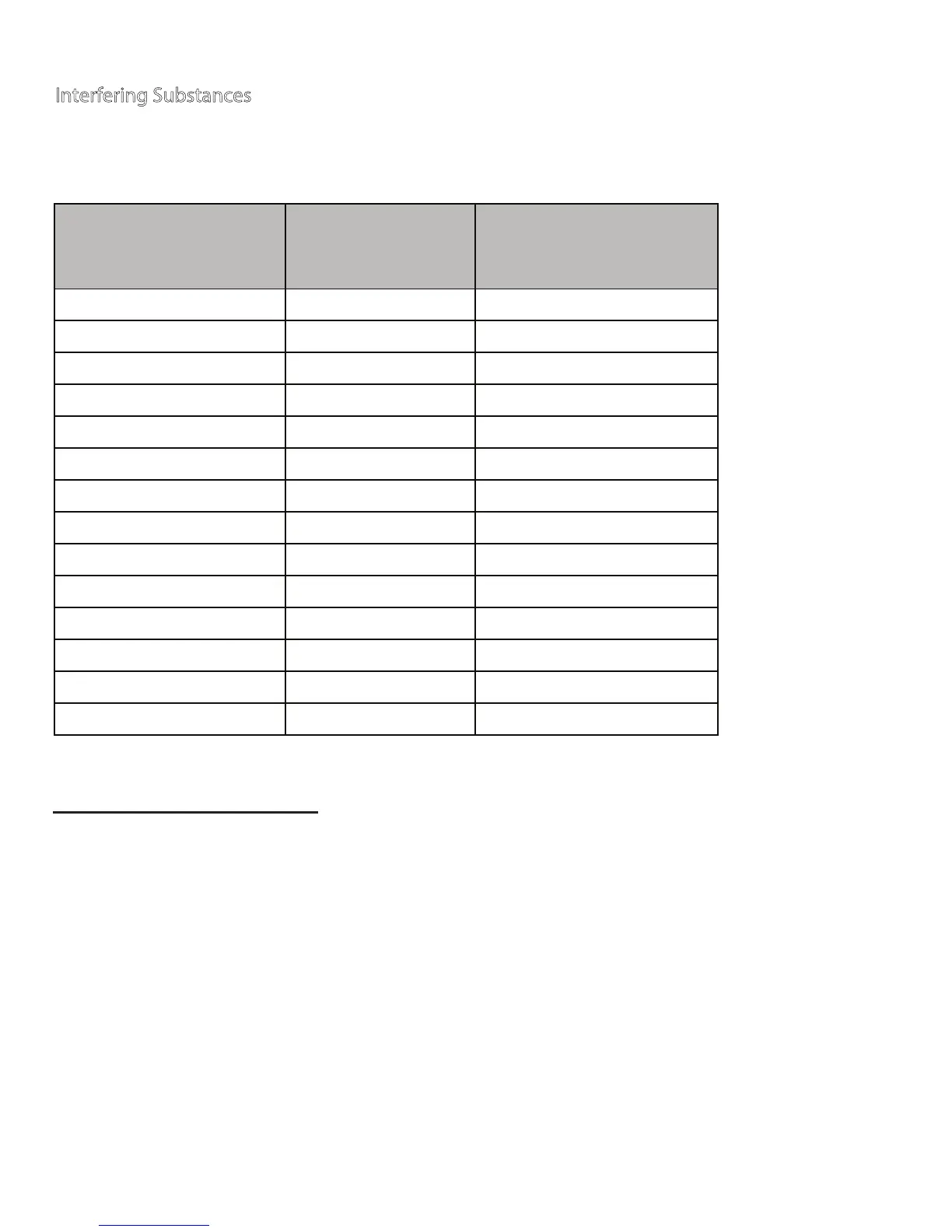
Substance
Limiting
Concentration
(mg/dL)
Therapeutic / Physiological
Concentration Range
(or Upper Limit) (mg/dL)
Acetaminophen 6.25 0.45 - 3
Ascorbic Acid 5 2
Bilirubin (Unconjugated)
20 0 - 2
Dopamine 1.25 0.03
Levo - Dopa 0.7 0.02 - 0.28
Methyl - Dopa 1.875 0.1 - 0.5
Glutathione Reduced 23 47 - 100 (intracellular)
Pralidoxime Iodide 5 ~10 ( IV Dose 500mg)
Tolazamide 12.5 1.6
Uric Acid 10 .2 - 8
Mannitol 5000 0.0128
Mannose 125 1.15
Xylose 3.125 N/A
*Na-Fluoride/K-Oxalate <250 250
* The NaF/Koxalate concentration is the standard concentration in a blood collection tube.
Interfering substances depend on the concentration. The interfering substances listed
below may produce elevated glucose test results (up to test concentration levels noted).
Interfering Substances
 Loading...
Loading...