37
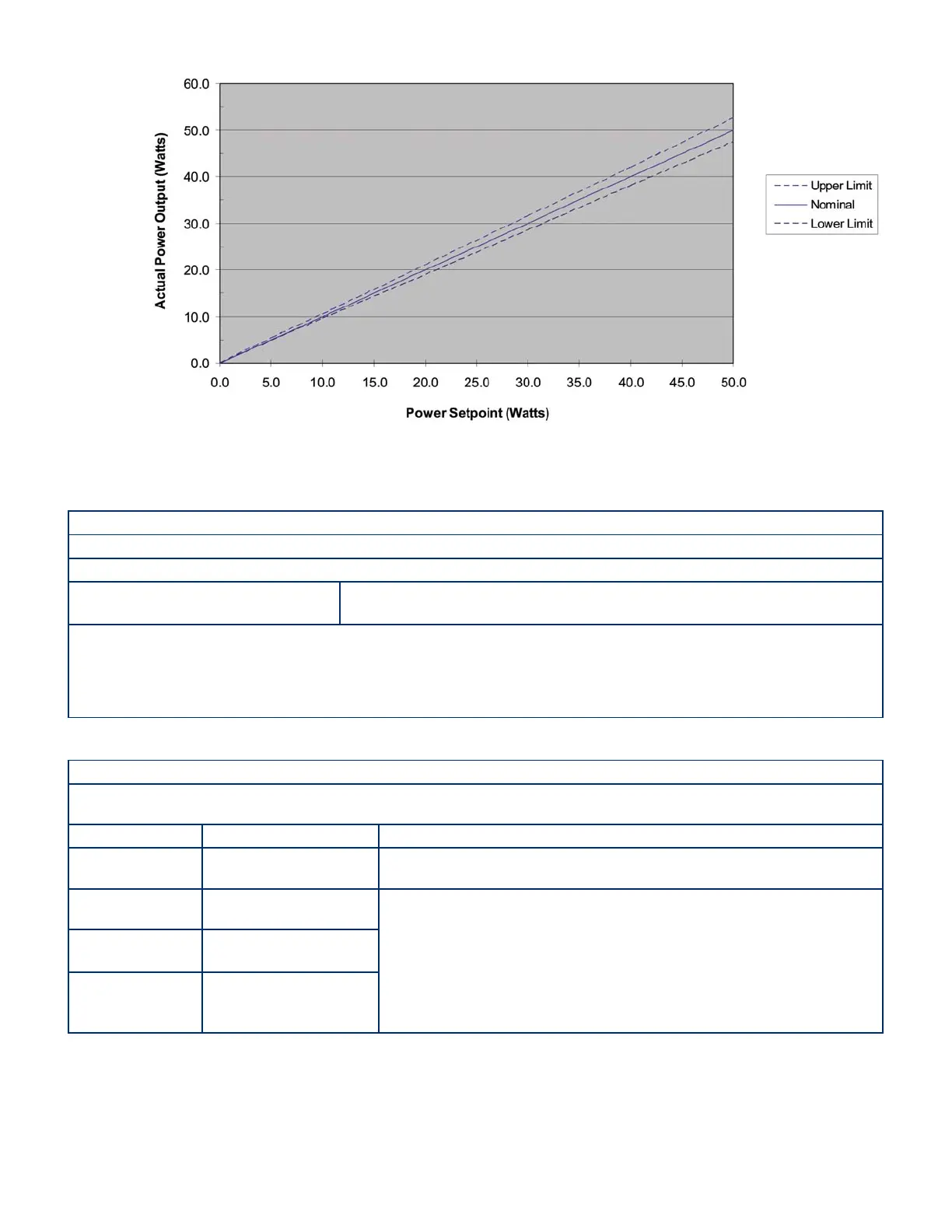
Figure 8-2 Set Power vs. Output Power
8.16 IEC Electrical Safety and EMC Specications
Table 8-1 IEC Electrical Safety Specications
Device Description
Class I, Debrillation proof Type CF Equipment, IPX0, not AP/APG
Mode of Operation: Continuous
Electrical Isolation • Leakage current conforms to IEC 60601-1
• Dielectric withstanding voltage conforms to IEC 60601-1
EMC Emissions and Susceptibility: The HALYARD* Pain Management System has been tested and found to comply with the limits for medical devices to the IEC 60601-1-
1-2:2007-03. These limits are designed to provide reasonable protection against harmful interference in a typical medical installation. This system generates, uses, and can
radiate radiofrequency energy and, if not installed and used in accordance with the instruction given below, may cause harmful interference to other devices in the vicinity.
However, there is no guarantee that interference will not occur in a particular installation. Portable and mobile RF communications equipment can aect medical electrical
equipment.
Table 8-2 IEC EMC Specications (Emissions)
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions
The HALYARD* Pain Management System is intended for use in the electromagnetic environment specied below. The customer or the user of the HALYARD* Pain
Management System should assure that it is used in such an environment.
Emissions Test Compliance Electromagnetic Environment – Guidance
RF emissions
CISPR 11
Group 1 The HALYARD* Pain Management System must emit electromagnetic energy in order to perform its
intended function. Nearby electronic equipment may be aected.
RF emissions
CISPR 11
Class A The HALYARD* Pain Management System is suitable for use in all establishments other than domestic and
those directly connected to the public low-voltage power supply network that supplies buildings used for
domestic purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage uctuations/
icker emissions
IEC 61000-3-3
Complies
Avanos Exhibit 2069 Page 37
Medtronic, Inc. v. Avanos Medical Sales, LLC
Case IPR2020-0089
 Loading...
Loading...