8 The MetaNeb® System Instructions for Use (174432 REV 10)
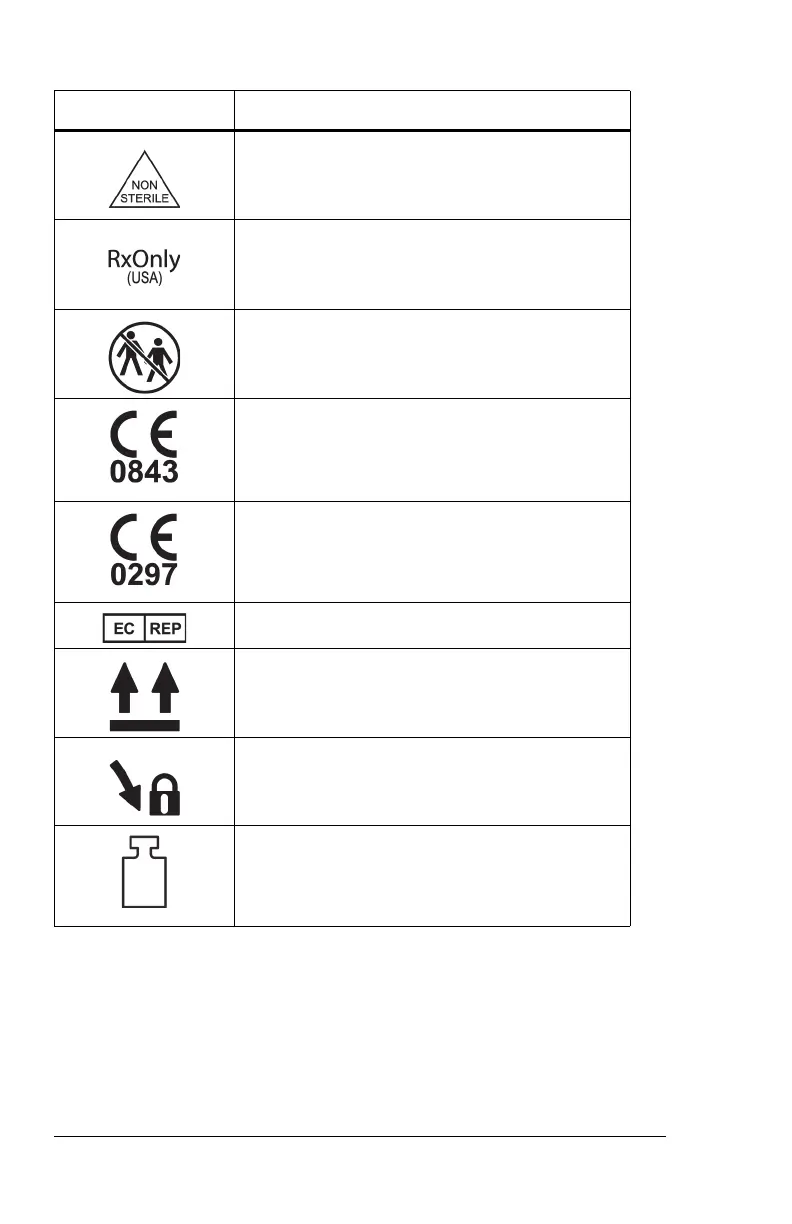
Symbols
Non-sterile product
By Prescription Only (USA only)
Single patient use product
The MetaNeb® System conforms to the
European Medical Device Directive
93/42/EEC
a
. Units made before September 14,
2018.
The MetaNeb® System conforms to the
European Medical Device Directive
93/42/EEC. Units made on and after Septem-
ber 14, 2018.
Authorized European Representative
Maintain the circuit tri-connector/bio-filter in
an upright position when in use.
Turn the circuit tri-connector/bio-filter
clockwise for secure fit with the control unit.
Mass of medical equipment including the
mobile stand, control unit, patient circuit, and
the maximum load permitted to put in the
basket.
a. The CE mark was first applied in 2014. The mark applies only to a
controller (Part number 186071).
Symbol Definition
 Loading...
Loading...