44 The MetaNeb® System Instructions for Use (174432 REV 10)
Particle Specifications
PARTICLE SPECIFICATIONS
The following specifications were established through performance tests
using an eight-stage cascade impactor at a flow rate of 28 liters per
minute (LPM) equipped with a USP <601> induction port throat. Three
device samples were tested with three runs each for a total of nine sample
points per each drug for a total of 27 data points. Aerosol was sampled
directly from the outlet.
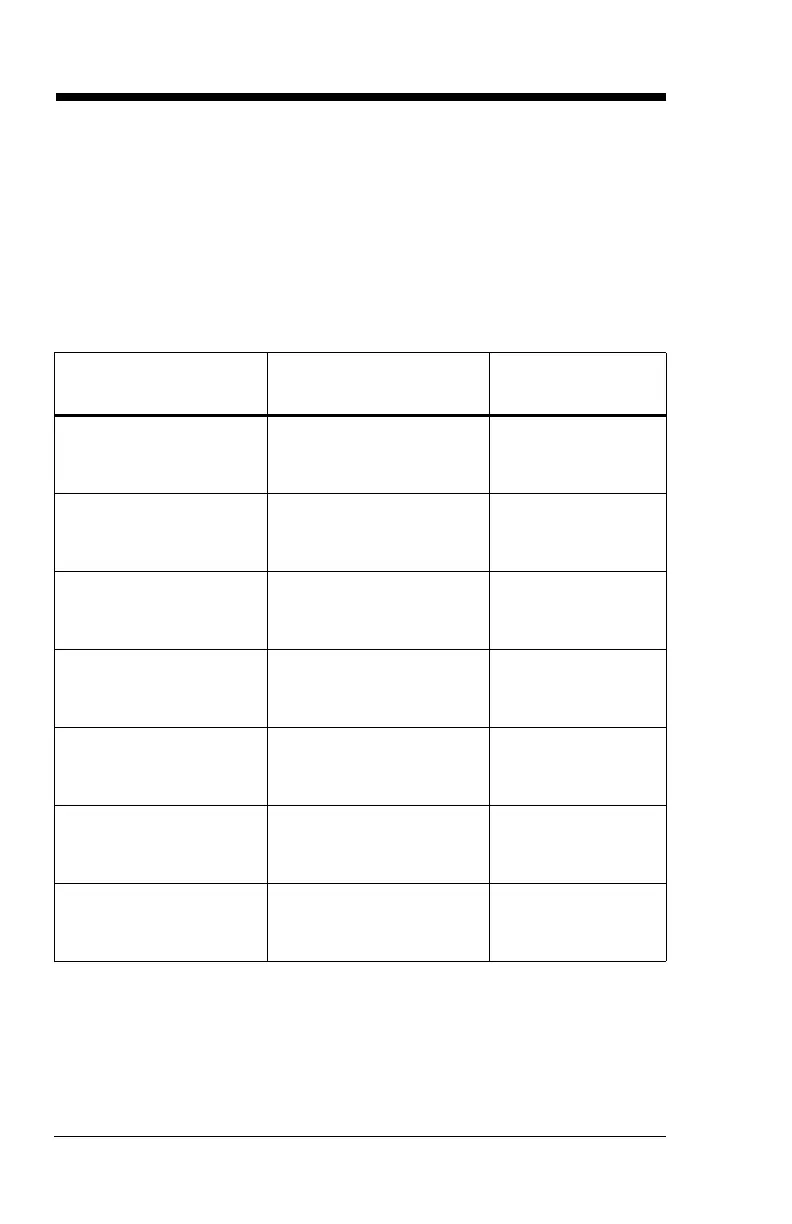
Aerosol Only Mode at 28 LPM
Particle Specifications with 95% Confidence Level
Coarse particles (due to oro-pharyngeal deposition) and ultra-fine
particles (due to exhalation) are not likely to deposit in the patient’s
airway and provide limited clinical benefit.
Particle
Characterization
Drug MetaNeb
MMAD (um) Albuterol
Ipratropium
Cromolyn
0.98-1.28
0.81-1.20
0.83-1.19
GSD 2.65-3.05
2.5-3.81
2.17-3.42
Total Dose Delivered by
Device ug
735.5-890.6
149.2-187
2567.6-3046.6
Total Respirable Dose
(0.5 - 5 um)
328.4-445.8
38.1-76.4
1554.4-1949.7
Coarse Particle Dose
>4.7 microns - ug
273.9-338.2
76-101.3
381.4-521.2
Fine Particle Dose
<4.7 microns - ug
427-587
59-99.9
2126.9-2584.7
Ultra-Fine Particle Dose
<1.0 Microns - ug
209.5-313.4
34.7-52
1032.1-1489.1
 Loading...
Loading...