23
Symbols
TUV Rheinland of North America is a Nationally Recognized Testing Laboratory (NRTL) in the United States and is accredited
by the Standards Council of Canada to certify electro-medical products with Canadian National Standards.
The product is designed not to become the ignition source in air and flammable anesthetic gas.
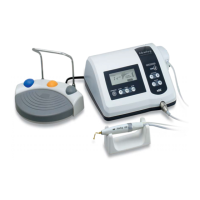
*Only Foot Control is AP equipment.
The EU directive 93/42/EEC was applied in the
design and production of this medical device.
Protected against the effects of continuous
immersion in dust and water.
Dispose of this device and its accessories via methods approved for electronic device and in compliance with the Directive
2002/96/CE.
Type BF applied part. Follow Operation Manual for use. Manufacturer
Authorised representative in the European community.
Autoclavable up to Max.135°C.
*for detail see Sterilization.
This product can be cleaned and disinfected with a Thermo-Disinfector.
Marking on the outside of Equipment or Equipment parts that include RF transmitters or that apply RF electromagnetic energy
for diagnosis or treatment.
Caution: U.S.Federal law restricts this device to sale by or on the order of a licensed physician.
Guidance and manufacturer's declaration - electromagnetic emissions
The VarioSurg is intended for use in the electromagnetic environment specified below. The customer or the user of the VarioSurg should assure that is used in such an environment.
Emissions test Compliance Electromagnetic environment - guidance
RF emissions
CISPR11/EN55011
Group 1 The VarioSurg uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to
cause any interference in nearby electronic equipment.
RF emissions
CISPR11/EN55011
class B The VarioSurg is suitable for use in all establishments other than domestic, and may be used in domestic establishments
and those directly connected to public low-voltage power supply network that supplies buildings used for domestic
purposes, provided the following warning is heeded:
Warning: VarioSurg is intended for use by healthcare professionals only. VarioSurg may cause radio interference or may
disrupt the operation of nearby equipment.
It may be necessary to take mitigation measures such as re-orienting or relocating the VarioSurg or shielding the location.
Harmonic emissions EN/IEC61000-3-2 class A
Voltage fluctuations/ flicker emissions
EN/IEC61000-3-3
Complies
Guidance and manufacturer's declaration - electromagnetic immunity
The VarioSurg is intended for use in the electromagnetic environment specified below. The customer or the user of the VarioSurg should assure that it is used in such an environment.
Immunity test EN/IEC60601 test level Compliance level Electromagnetic environment - guidance
Electrostatic discharge (ESD)
EN/IEC61000-4-2
±6kV contact
±8kV air
±6kV contact
±8kV air
Floors should be wood, concrete or ceramic tile. If floors are
covered with synthetic material, the relative humidity should
be at least 30%.
Electrical fast transient/burst
EN/IEC61000-4-4
±2kV for power supply lines
±1kV for input/output
±2kV for power supply lines
±1kV for input/output
Mains power quality should be that of a typical commercial or
hospital environment.
Surge
EN/IEC61000-4-5
±1kV line to line
±2kV lines to earth
±1kV line to line
±2kV lines to earth
Mains power quality should be that of a typical commercial or
hospital environment.
Voltage dips, short interruptions and
voltage variations on power supply input
lines
EN/IEC61000-4-11
<5% Ut (>95% dip in Ut)
for 0.5 cycle
40% Ut (60% dip in Ut)
for 5 cycles
70% Ut (30% dip in Ut)
for 25 cycles
<5% Ut (>95% dip in Ut)
for 5 sec
<5% Ut (>95% dip in Ut)
for 0.5 cycle
40% Ut (60% dip in Ut)
for 5 cycles
70% Ut (30% dip in Ut)
for 25 cycles
<5% Ut (>95% dip in Ut)
for 5 sec
Mains power quality should be that of a typical commercial
or hospital environment. If the user of the VarioSurg requires
continued operation during power mains interruptions, it
is recommended that the VarioSurg be powered from an
uninterruptible power supply or a battery.
Power frequency (50/60Hz)
magnetic field
EN/IEC61000-4-8
3 A/m 3 A/m Power frequency magnetic fields should be at levels
characteristic of a typical location in a typical commercial or
hospital environment.
NOTE: Ut is the a.c. mains voltage prior to application of the test level.
 Loading...
Loading...