40
N6
41
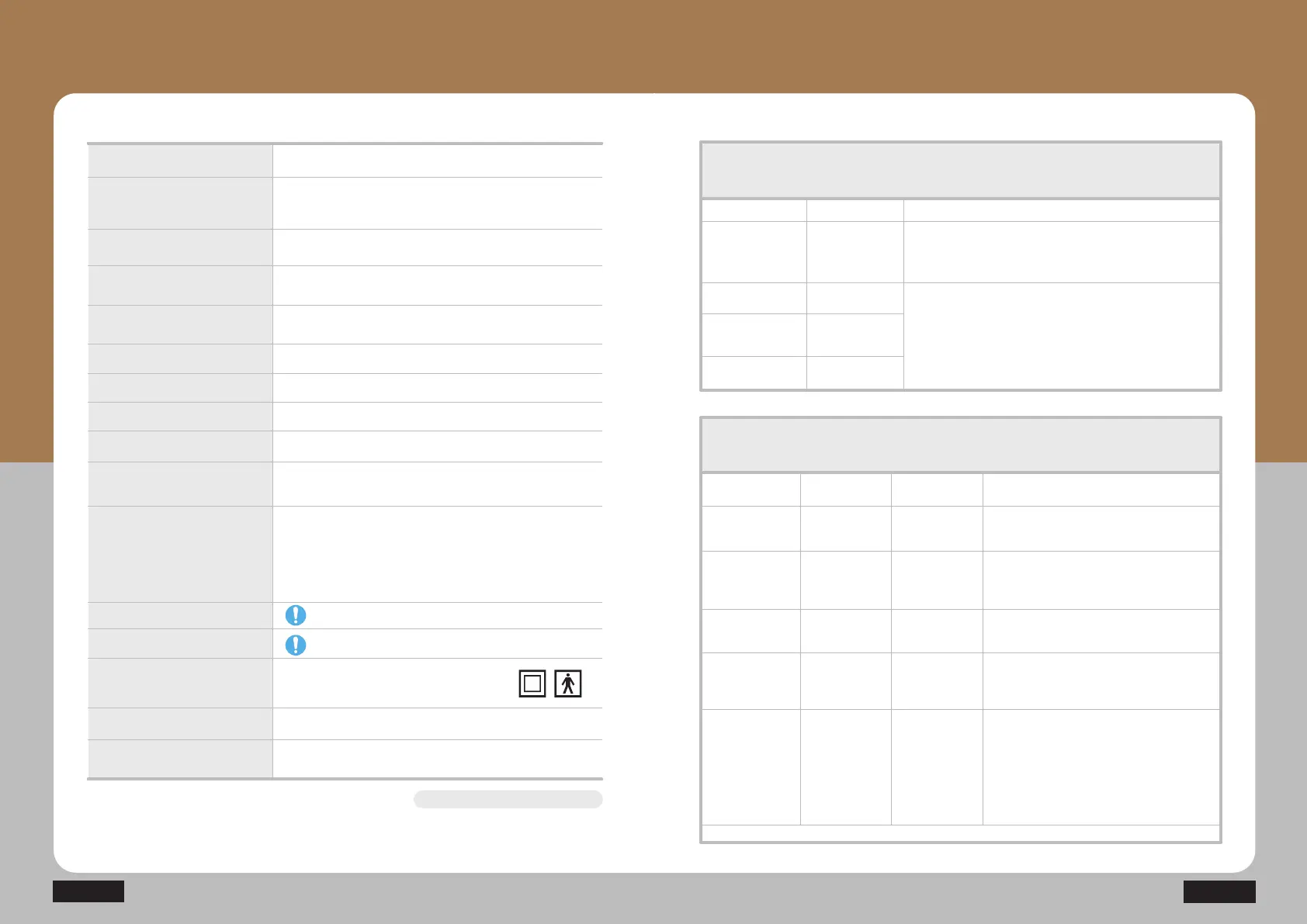
EMC Guidance and manufacturer’s declaration
Electromagnetic emission
The Model N6 (Combinational simulator) is intended for use in the electromagnetic
environment specified below. The customer or the user of N6 (Combinational stimulator)
should assure that it is used in such an environment.
RF emissions
CISPR 11
The Model N6 (Combinational simulator) uses RF energy
only for its internal function. Therefore. Its RF emissions are
very low and are not likely to cause any interference in nearby
electronic equipment.
Group 1
RF emissions
CISPR 11
The Model N6 (Combinational simulator) is suitable for use
in all establishments, including domestic establishments and
those directly connected to the public low-voltage power
supply network that supplies buildings used for domestic
purposes.
Class A
Harmonics
emission
IEC 61000-3-2
Voltage fluctuation
IEC 61000-3-3
A
Complies
Emission test Compliance Electromagnetic environment – guidance
Electromagnetic immunity
The Model N6 (Combinational stimulator) is intended for use in the electromagnetic
environment specified below. The customer or the user of N6 (Combinational stimulator)
should assure that it is used in such an environment.
Electrostatic
Discharge (ESD)
IEC 61000-4-2
Electrical fast
Transient /burst
IEC 61000-4-4
6kV Contact
8kV Air
6kV Contact
8kV Air
Floors should be wood, concrete or ceramic
tile.
If floors are covered with synthetic material,
the relative humidity should be at least 30%.
Mains power quality should be that of a typical
commercial or hospital environment.
Mains power quality should be that of a typical
commercial or hospital environment.
Power frequency magnetic fields should be
at levels characteristic of a typical location
in a typical commercial or hospital environment.
Main power quality should be that of a typical
commercial or hospital environment. If the user
of the Model. N6 (Combinational stimulator)
requires continued operation during power mains
interruptions. It is recommended that the Model
N6 (Combinational stimulator) be powered from
an uninterruptible power supply or a battery.
2kV for power
supply lines
1kV for input
/output lines
2kV for power
supply lines
1kV for input
/output lines
Surge
IEC 61000-4-5
1kV differential
mode 2KV
common mode
1kV differential
mode 2KV
common mode
Power frequency
(50/60Hz)
Magnetic field
IEC 61000-4-8
Voltage dips,
short Interruptions
and Voltage
variations on ower
supply input lines
IEC 61000-4-11
* Note: UT is the a.c. mains voltage prior to application of the test level.
3.0 A/m 3.0 A/m
<5% UT(>95% dip
in UT)for 0.5 cycle
40% UT(60% dip
in UT) for 5 cycle
70% UT(30% dip
in UT) for 25 cycle
<5% UT(<95% dip
in UT) for 5 s
5% UT(>95% dip
in UT) for 0.5 cycle
40% UT(60% dip
in UT) for 5 cycle
70% UT(30% dip
in UT) for 25 cycle
<5% UT(<95% dip
in UT) for 5 s
Immunity test
IEC 60601
Test level
Compliance
level
Electromagnetic environment-guidance
EMC Guidance and
manufacturer’s declaration
Combinational Stimulator N6
NUGA MEDICAL CO.,LTD.
185, Jiraeul-ro, Jijeong-myeon, Wonju-si,
Gangwon-do, Korea
No. 1478
No. 15-146
Marked on the product
50.54 kg
1925 X 580 X 516 (mm)
Refer to the user manual
Refer to the user manual
Help to muscle relaxation, blood circulation and
alleviate muscular pains
This device should not be used by a pregnant woman
or a patient with malignant tumor, high fever, heart
disease (pacemaker implant), perceptual disorder, skin
problem in the target area, or spondylolysis
AC 230 V~, 50 Hz
370 W
Class II equipment, type BF applied part
47 minutes
5 year
Product name and model name
Manufacturer
License number of
manufacturing business
License number for the
manufacturing article
Date of manufacture /
number of manufacture
Product weight
Product size
Cautions during use
Using method
Intended Use
Contraindications
Electric rating
Power consumption
Type and class of
protection for electric shocks
Maximum using time
Life-time of product
This product is a medical device.
Product Specifications
 Loading...
Loading...