Symbols and Abbreviations
The packaging material, the identification plate on the instrument and the manual may contain the following symbols or
abbreviations:
Please consult instructions for use
Caution (refer to accompanying documents). Please refer to safety-related notes in the manual
accompanying this instrument.
Store at
Manufacturer
Date of manufacture
RoHS
Approvals
The Urisys analyzer (serial# and higher) meets the requirements laid down in:
Directive //EU of the European Parliament and of the Council of June on the restriction
of the use of certain hazardous substances in electrical and electronic equipment.
Global trade item number
Catalogue number
For in vitro diagnostic use
This product fulfils the requirements of Directive //EC on in vitro diagnostic medical devices.
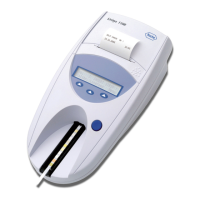
 Loading...
Loading...