0210-100-700 Rev-T PL
www.stryker.com 83
Parametry techniczne
Model: Seria REF 0210-XXX-000 Seria REF 0211-XXX-000
Opis:
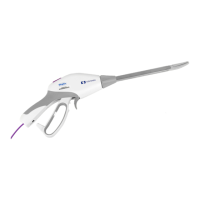
Uchwyt, zasilacz na baterie
(Handpiece, battery power pack)
Uchwyt, zasilacz akumulatorowy
(Handpiece, rechargeable power pack)
Wymiary (uchwyt):
Długość 18,4 cm
Szerokość 3,1 cm
Wysokość 2,7 cm
Waga: 0,78 kg (uchwyt z zasilaczem na baterie) 0,51 kg (tylko uchwyt)
Długość drenów:
2,82 m (irygacja)
3,05 m (ssanie)
Zasilanie elektryczne: 12 V , osiem baterii alkalicznych AA, 1,5 V, zasilanie wewnętrzne
Zasilacz akumulatorowy InterPulse (InterPulse Rechargeable Power
Pack), 7,9 V
Oznaczenie CE:
Seria REF 0210-XXX-XXX (również z przyrostkiem E), z wyjątkiem: REF
0210-100-000S4, REF 0210-114-000S5, REF 0210-165-000, REF 0210-218-
100, REF 0210-900-000 oraz REF 0210-918-000.
Tylko REF 0211-100-000, REF 0211-100-000E, REF 0211-110-000,
REF 0211-110-000E, REF 0211-022-000 oraz REF 0211-024-000.
Stopień ochrony
urządzenia przed
penetracją czynników
zewnętrznych (IP):
IPX0
Rodzaj sprzętu: Część mająca bezpośredni kontakt z ciałem pacjenta typu B
Tryb pracy: Ciągły
Certyfikat bezpieczeństwa produktu:
Dotyczy wyłącznie serii
REF 0211-1XX-000 z
przyrostkiem E lub bez
niego, serii REF 0211-2XX-
XXX z przyrostkiem E lub
bez niego oraz REF 0211-
022-000:
Canadian Standards Association (CSA) International
Canadian Standards Association (CSA)
CAN/CSA-C22.2 No. 601.1-M90, Medical Electrical Equipment — Part 1: General Requirements for Safety (Adopted IEC 60601-1 2d Ed
[90])
CAN/CSA C22.2 601.1S1-94, Supplement No 1-94 to CAN/CSA C22.2 601.1-M90
CAN/CSA C22.2 601.1B-98, Amendment 2 to CAN/CSA C22.2 601.1-M90
Underwriters Laboratories (UL)
UL 60601-1 (1st Ed), Medical Electrical Equipment — Part 1: General Requirements for Safety
Dotyczy REF 0210-0XX-
XXX, REF 0210-1XX-XXX,
REF 0210-2XX-XXX, REF
0210-9XX-XXX, wraz ze
wszystkimi częściami E
oraz S, a także REF
0207-050-190:
Canadian Standards Association (CSA) International
International Electrotechnical Commission (IEC)
IEC 60601-1 A1: R:2012 (3.1 Ed), Medical Electrical Equipment — Part 1: General Requirements for Basic Safety and Essential
Performance; Corrigendum 1 (2006); Corrigendum 2 (2007); Amendment 1 (2012)
Canadian Standards Association (CSA)
CAN/CSA-C22.2 No. 60601-1:08, Medical Electrical Equipment — Part 1: General Requirements for Basic Safety and Essential
Performance (Adopted IEC 60601-1:2005 + CORR. 1)
CAN/CSA-C22.2 No. 60601-1:08 TC 2:2011 (Corrigendum 2), Medical Electrical Equipment — Part 1: General Requirements for Basic
Safety and Essential Performance (Adopted IEC 60601-1:2005 + CORR. 1)
CAN/CSA-C22.2 No. 60601-1:14, Medical Electrical Equipment — Part 1: General Requirements for Basic Safety and Essential
Performance (3.1 Ed) (Adopted IEC 60601-1:2005 Edition 3.0 + AMENDMENT 1, 2012-07, MOD)
CAN/CSA-C22.2 No. 60601-1-6:11, Medical Electrical Equipment – Part 1-6: General Requirements for Basic Safety and Essential
Performance – Collateral Standard: Usability
American National Standards Institute (ANSI)/Association for the Advancement of Medical Instrumentation (AAMI)
ANSI/AAMI ES60601-1:2005 (IEC 60601-1:2005, MOD), Medical Electrical Equipment — Part 1: General Requirements for Basic Safety
and Essential Performance
ANSI/AAMI ES60601-1:2005/C1:2009, Medical Electrical Equipment — Part 1: General Requirements for Basic Safety and Essential
Performance – Corrigendum C1
ANSI/AAMI ES60601-1:2005/A2:2010, Medical Electrical Equipment — Part 1: General Requirements for Basic Safety and Essential
Performance – Amendment A2
ANSI/AAMI ES60601-1:2005/(R)2012, AND C1:2009 AND A2:2010(R)2012 (Consolidated text — Edition 3.1), Medical Electrical
Equipment — Part 1: General Requirements for Basic Safety and Essential Performance (IEC 60601-1:2005+A1:2012, MOD)
European Committee for Electrotechnical Standardization (CENELEC)
CENELEC/EN 60601-1:2006/A1:2013 (3.1 ED), Medical Electrical Equipment – Part 1: General Requirements for Basic Safety and
Essential Performance – incorporates Amendment A12
0000004059, Rev. T Effective Date: Sep 26, 2016 2:47:57 PM
Print Date: Nov 02, 2016 02:57:27 PM

 Loading...
Loading...