73
15. EMC information – P90
Warnings:
Medical Electrical Equipment needs special precautions regarding EMC and needs to be
installed and put into service according to the EMC information provided in the Accompanying
Documents.
Portable and Mobile RF Communications Equipment can affect Medical Electrical Equipment.
The use of accessories and cables other than those specified, with the exception of those sold
by the manufacturer as replacement parts for internal components, may result in increased
emissions or decreased immunity of the equipment or system.
The Tanning device should not be used adjacent to or stacked with other equipment and that
if adjacent or stacked use is necessary, the Tanning device should be observed to verify normal
operation in the configuration in which it will be used.
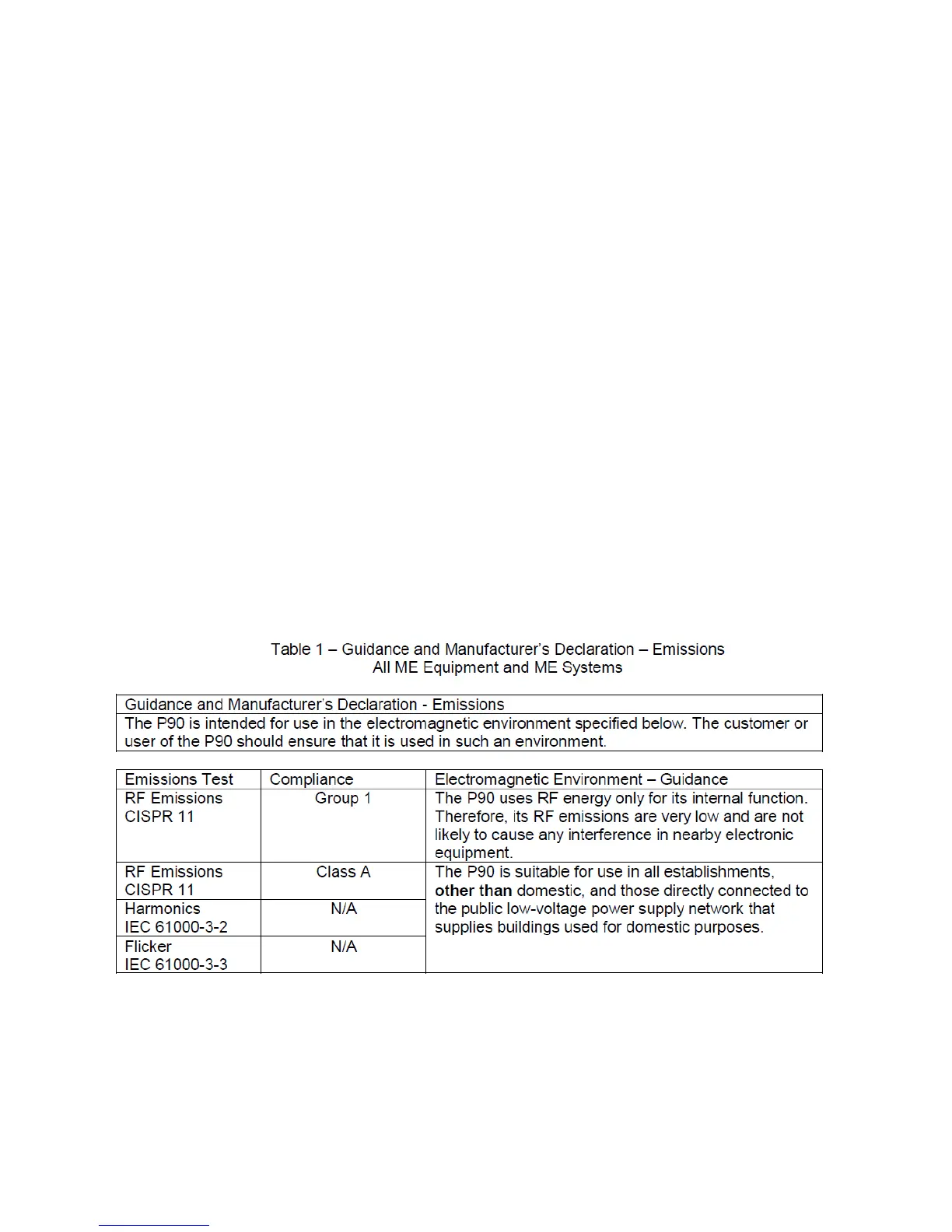
15.1.1 Table 1: Guidelines and manufacturer's
declaration – Electromagnetic emissions
The tanning device has been designed for use in the electromagnetic environment
specified below. The device operator must ensure that it is used in such an
environment
 Loading...
Loading...