IM 12B6B3-E-H
Glossary
GLOSSARY
pH (-log [H+] ) This is a logarithmic function of the Hydrogen ion activity (concentration). This pro-
vides a quick indication of the acidic or alkaline behavior of a dilute solution.
Normally measured on a scale of 0-14 pH where low numerical values are acidic (0
is approximately 1 Normal acid) and high numbers are alkaline (14 is approximately
1 Normal NaOH). The neutral point is pH 7.
Defined by Nernst in the following equation: E = Eo + RT/nF x Ln [H+]
E = measured potential
R = gas constant
T = absolute temperature
n = valence
F = Faraday number
Ln = Napierian logarithm
[H+] = activity of the Hydrogen ion
Eo = Reference potential
ORP Oxidation reduction potential is a measure of oxidizing power of a solution. The
greater the milliVolt value with a negative polarity, the greater the oxidizing power.
Reducing power is indicated by positive values of mV.
rH This is a composite value that indicates the oxidizing power of a solution compen-
sating for the influence of the acid or alkaline components. The scale is 0-55 rH,
where oxidizing solutions provide the highest readings.
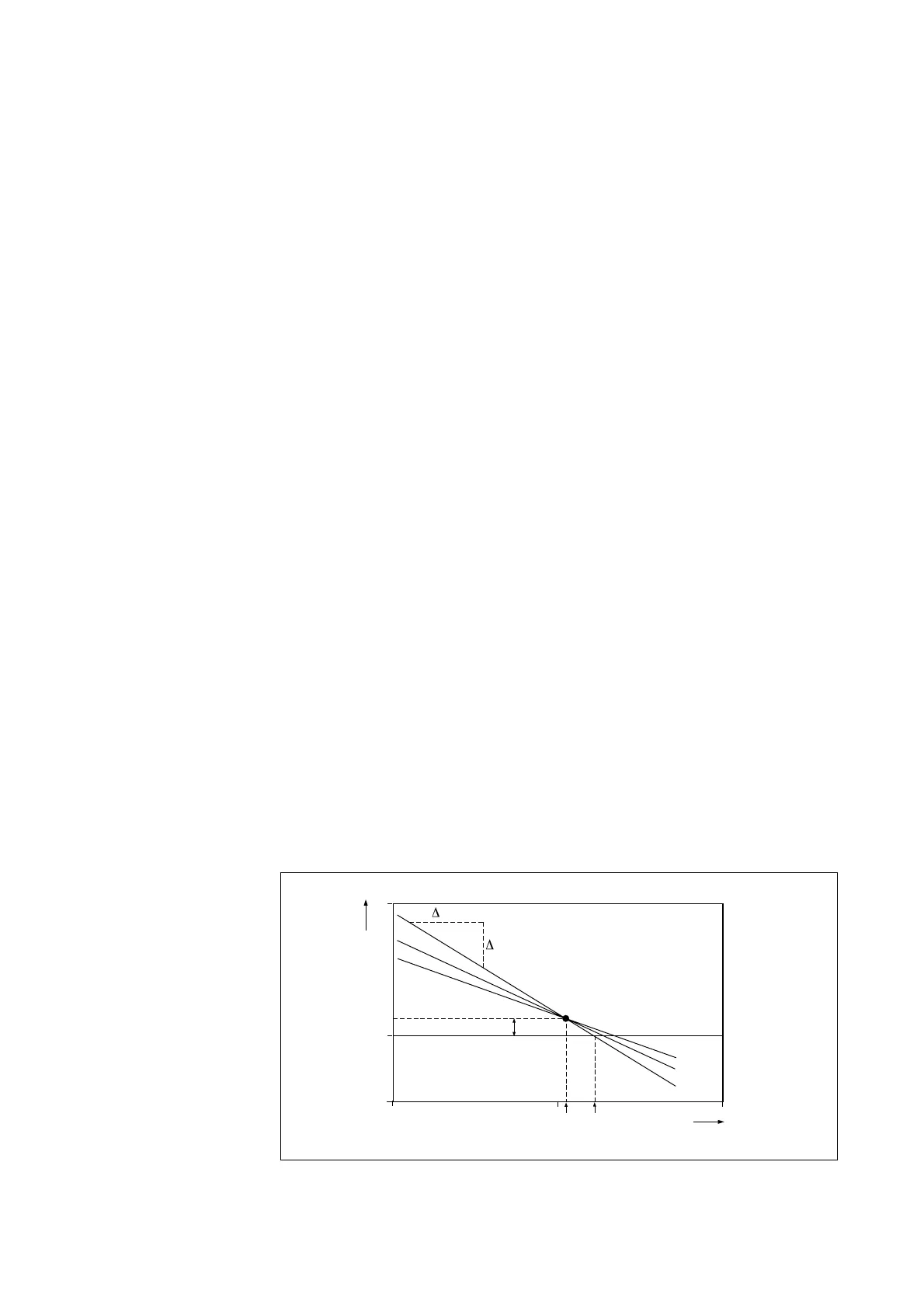
Asymmetry potential This is the difference between the isothermal point of intersection and the zero
point.
Slope This is the sensitivity of the pH electrode (mV/pH) usually expressed as a % of the
theoretical value (Nernst).
ITP This is the isothermal point of intersection. This is the value in pH at which the
temperature response of the system is at a null point. In other words, the point of
intersection of the temperature lines on a graph of millivolts vs pH. This point is
critical to the correct operation of the temperature compensation circuitry.
Zero point This is the value of pH at which the electrode combination yields 0 mV as an out-
put.

 Loading...
Loading...