SECTION
C
21
Regulatory Review and Guidelines
for QA/QC Protocols
COMPETENCY CHECKLIST
Joint Commission on
Accreditation of Healthcare
Organizations (JCAHO)
Clinical and Laboratory
Standards Institute (CLSI)
(Formerly NCCLS)
Clinical Laboratory
Improvement
Amendments (CLIA)
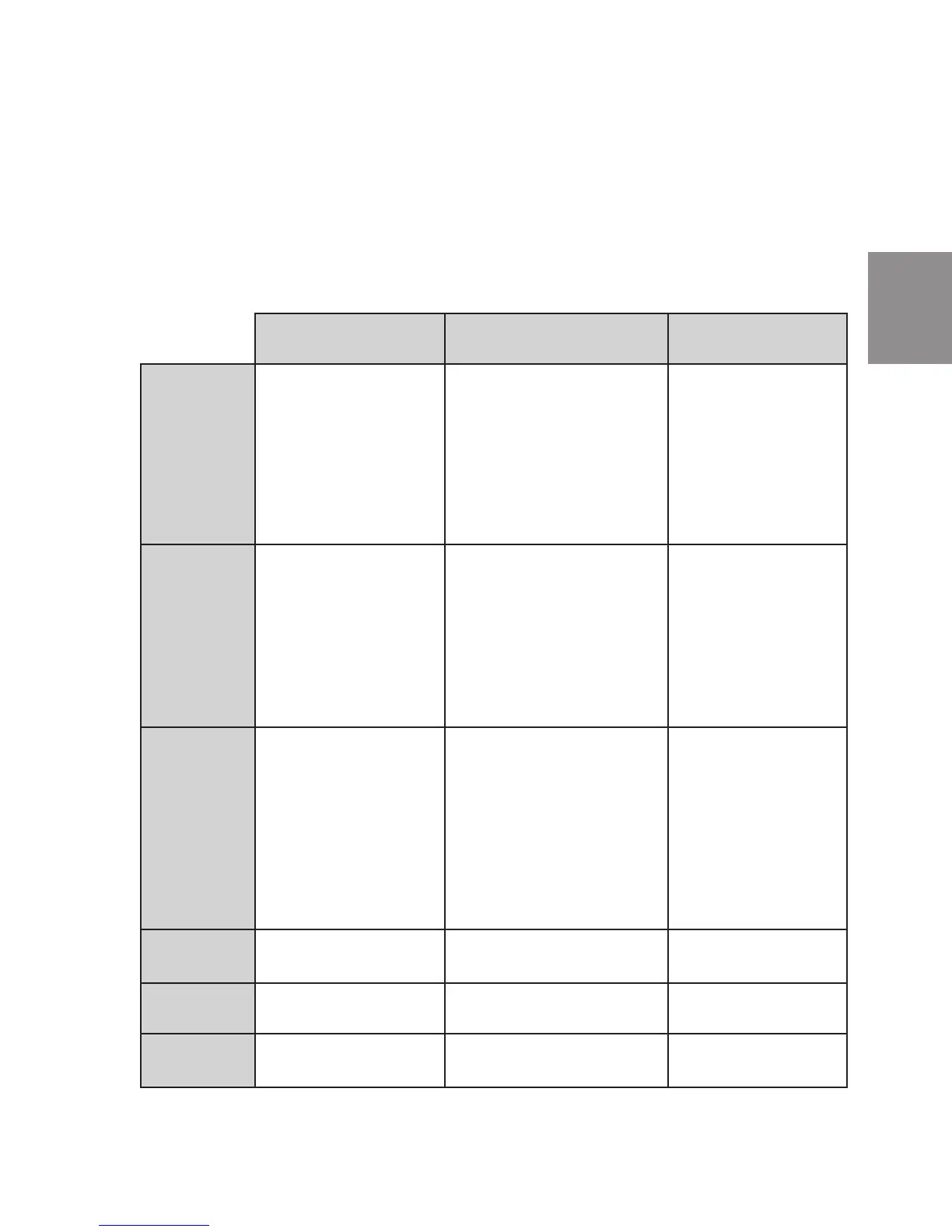
Personnel/Training • Staff members can describe
or demonstrate their roles and
responsibilities (HR.2.20).
• Participate in ongoing
in-services.
• Education is documented
(HR.2.30).
• Competence to perform job
responsibilities is assessed,
demonstrated, and maintained
(HR.3.10).
• Formal training and written
examination.
• Follow manufacturer’s
instructions.
Quality Assurance • Current and complete policies
are available to the person
performing the test.
• Written policies and
procedures for: specimen
collection,identication,and
requiredlabeling;specimen
preservation;instrument
calibration;qualitycontroland
remedialaction;equipment
performanceevaluation;
test performance.
• Follow manufacturer’s instructions
for calibration.
• Written procedure manual.
• Manual contains principles of
operation, reagents/equipment,
calibration, quality control, stepwise
procedure, reporting results,
procedure limitations, references,
supplemental material, review
and updates.
• Follow manufacturer’s
instructions.
Quality Control • Written quality control plan
thatspecieshowprocedures
will be controlled.
• At the least, perform as
frequently as recommended
by manufacturer.
• Should include two levels of
control solution.
• At the least, quality control
procedures are performed
once each day on each
instrument used for resident
testing (PC.16.50).
• On each day of use, two control
solutions(Level1and2)shouldbe
performed per instrument.
•Priortothersttestoftheday,
each operator is to perform a quality
control test.
• Additional checks should be
performed when:
• A new bottle of strips is opened.
• Each time a reagent lot is changed.
• To ensure the strips and meter
are functioning properly.
• Follow manufacturer’s
instructions.
Linearity/Calibration •Linearitynotcoveredin
the standards.
•Initiallyandasspeciedby
manufacturer or as required by
government or accrediting bodies.
• Follow manufacturer’s
instructions.
Prociency Training •Notspecied. • Participate in program that
meets accreditation, federal
and state regulations.
•Notspecied.
Inspections • Currently triennial (APP-5). • Not a certifying agency. • Not subject to routine survey.
• Conducted only when
authorized by the RO.
ARKRAY provides this checklist as a guideline only. These guidelines are subject to change by the
regulating body at any time. Utilize this checklist in accordance with your facility’s policy.
 Loading...
Loading...









