neoprobe
TM
GDS Operation Manual
AW-000639 Page 34 of 38
EMC Requirements in Accordance with EN 60601-1-2:2007
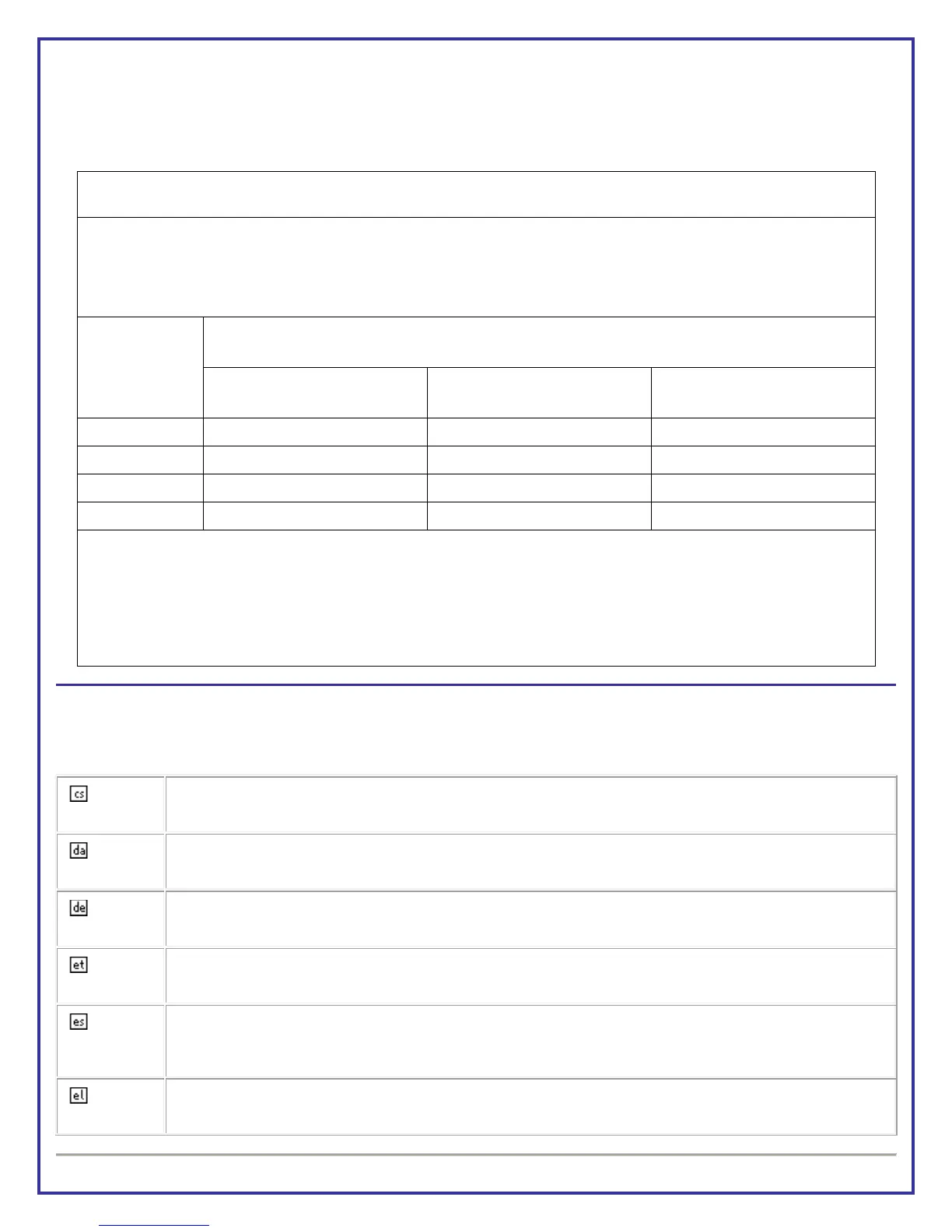
Table 206
Recommended separation distance between portable and mobile RF communications equipment and the Model 2300
Gamma Detection System equipment
The Model 2300 Gamma Detection System equipment is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the Model 2300 Gamma Detection System equipment can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile communications equipment
(transmitters) and the Model 2300 Gamma Detection System equipment as recommended below, according to the maximum output
power of the communications equipment.
Rated maximum
output power of
transmitter
W
Separation distance according to frequency of transmitter
m
150KHz to 80MHz
d = 1.17 * √ P
80MHz to 800 MHz
d = 1.17 * √ P
800 MHz to 2.5 GHz
d = 2.33 * √ P
For transmitters rated at a maximum output power not listed above, the recommended separation distance (d) in meters (m) can be
estimated using the equation applicable to the frequency of the transmitter, where (P) is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80MHz and 800 MHz, the separation distance for the higher frequency applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects and people.
Declaration of Conformance:
Hereby, Devicor Medical Products, Inc. declares that this “Wireless Probe” is in compliance with the essential requirements and other
relevant provisions of Directive 1999/5/EC.
Devicor Medical Products, Inc. tímto prohlašuje, že tento “Wireless Probe” je ve shodě se základními požadavky a
dalšími příslušnými ustanoveními směrnice 1999/5/ES.
Undertegnede Devicor Medical Products, Inc. erklærer herved, at følgende udstyr “Wireless Probe” overholder de
væsentlige krav og øvrige relevante krav i direktiv 1999/5/EF.
Hiermit erklärt Devicor Medical Products, Inc., dass sich das Gerät “Wireless Probe” in Übereinstimmung mit den
grundlegenden Anforderungen und den übrigen einschlägigen Bestimmungen der Richtlinie 1999/5/EG befindet.
Käesolevaga kinnitab Devicor Medical Products, Inc. seadme “Wireless Probe” vastavust direktiivi 1999/5/EÜ
põhinõuetele ja nimetatud direktiivist tulenevatele teistele asjakohastele sätetele.
Por medio de la presente Devicor Medical Products, Inc. declara que el “Wireless Probe” cumple con los
requisitos esenciales y cualesquiera otras disposiciones aplicables o exigibles de la Directiva 1999/5/CE.
ΜΕ ΤΗΝ ΠΑΡΟΥΣΑ Devicor Medical Products, Inc. ΔΗΛΩΝΕΙ ΟΤΙ “Wireless Probe” ΣΥΜΜΟΡΦΩΝΕΤΑΙ
ΠΡΟΣ ΤΙΣ ΟΥΣΙΩΔΕΙΣ ΑΠΑΙΤΗΣΕΙΣ ΚΑΙ ΤΙΣ ΛΟΙΠΕΣ ΣΧΕΤΙΚΕΣ ΔΙΑΤΑΞΕΙΣ ΤΗΣ ΟΔΗΓΙΑΣ
Artwork No: AW-000639 Revision: A Artwork Status: Released Lifecycle Name: Artwork
PCO No: N/A ECN No: ECN-000214 Release Date: 10/09/2012 Effective Date: 10/09/2012

 Loading...
Loading...