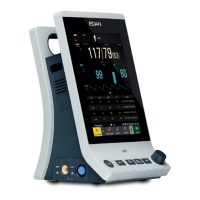
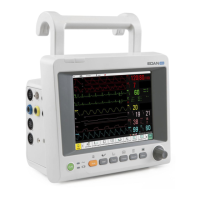
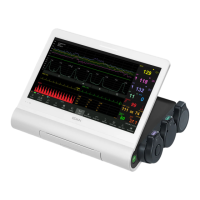
What to do if my EDAN Monitor has no display?
- KKaren ChavezAug 20, 2025
If your EDAN Monitor isn't displaying anything, there could be a few reasons. First, ensure the power cable is securely connected and check the fuse. Also, check the battery. If the fuse is blown, replace it. If the battery is low, connect the monitor to AC power.