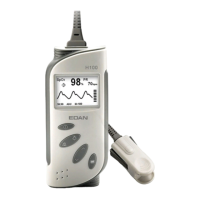
Why is the SpO2 or PR value unstable on my EDAN H10?
- CClifford MartinAug 6, 2025
If the SpO2 or PR value on your EDAN Medical Equipment is unstable, it might be because your finger isn't inserted deeply enough into the device. Try re-applying the sensor, ensuring your finger is properly positioned. Alternatively, instability can occur if your finger is trembling or you are moving; in this case, please remain still during measurement.