21
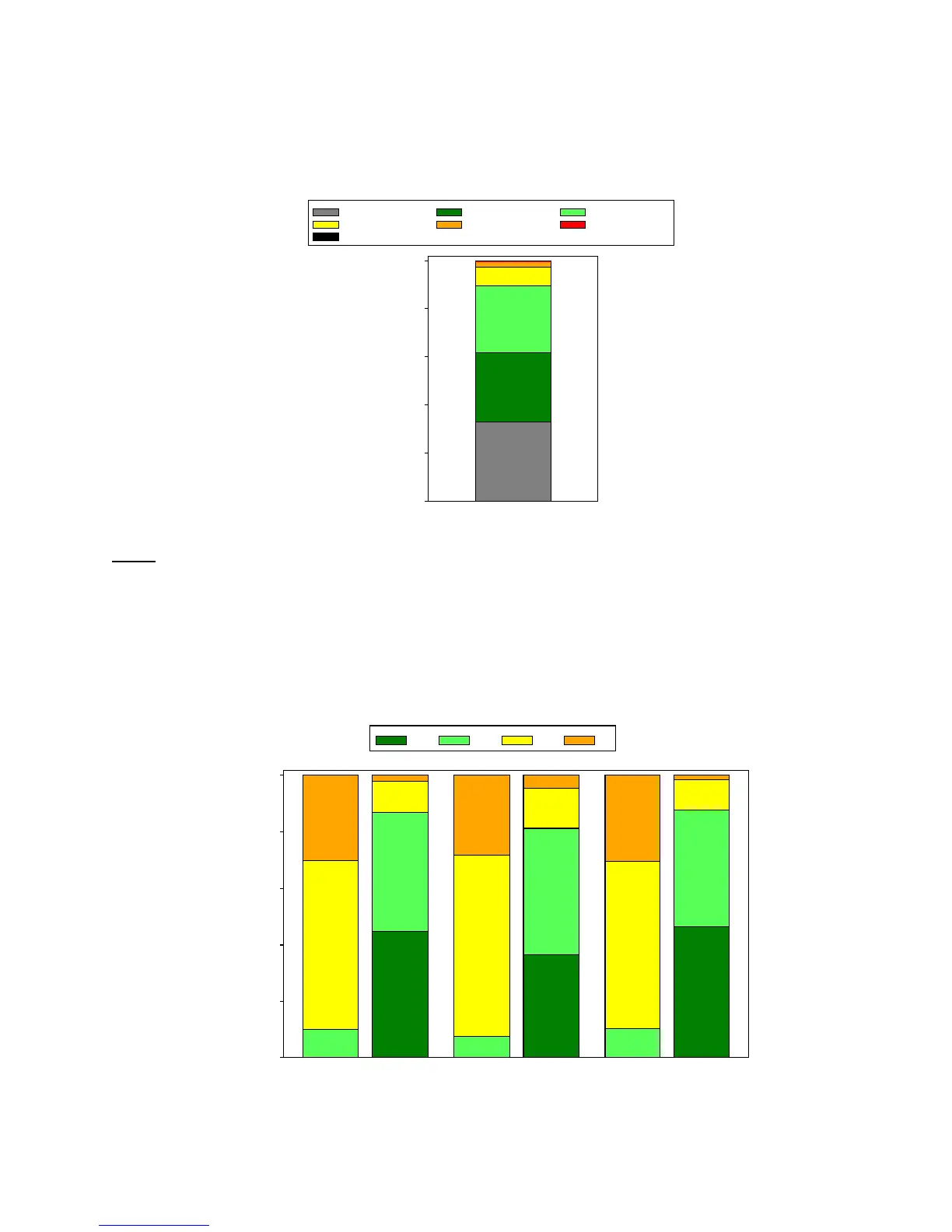
The proportion of patients with aortic paravalvular leak (PVL) ≥ moderate was 2.9% at 30 days, as shown
in Figure 9.
Aortic Paravalvular Leak
NYHA
The NYHA class by visit is shown in Figure 10. For all patients, the mean NYHA class was 3.2 ± 0.6 at
baseline and 1.7 ± 0.7 at 30 days.
NYHA Class by Visit
None
Trace Mild
Mild-Moderate Moderate Moderate-Severe
Severe
Percentage
0
20
40
60
80
100
30 Day
33%
29%
28%
8%
1 2
3 4
Percent
0
20
40
60
80
100
All Patients Transapical/Transaortic Transfemoral
Baseline 30 Day Baseline 30 Day
Baseline 30 Day
10%
60%
30%
45%
42%
11%
8%
64%
28%
36%
45%
14%
5%
10%
59%
31%
46%
42%
11%
 Loading...
Loading...