35
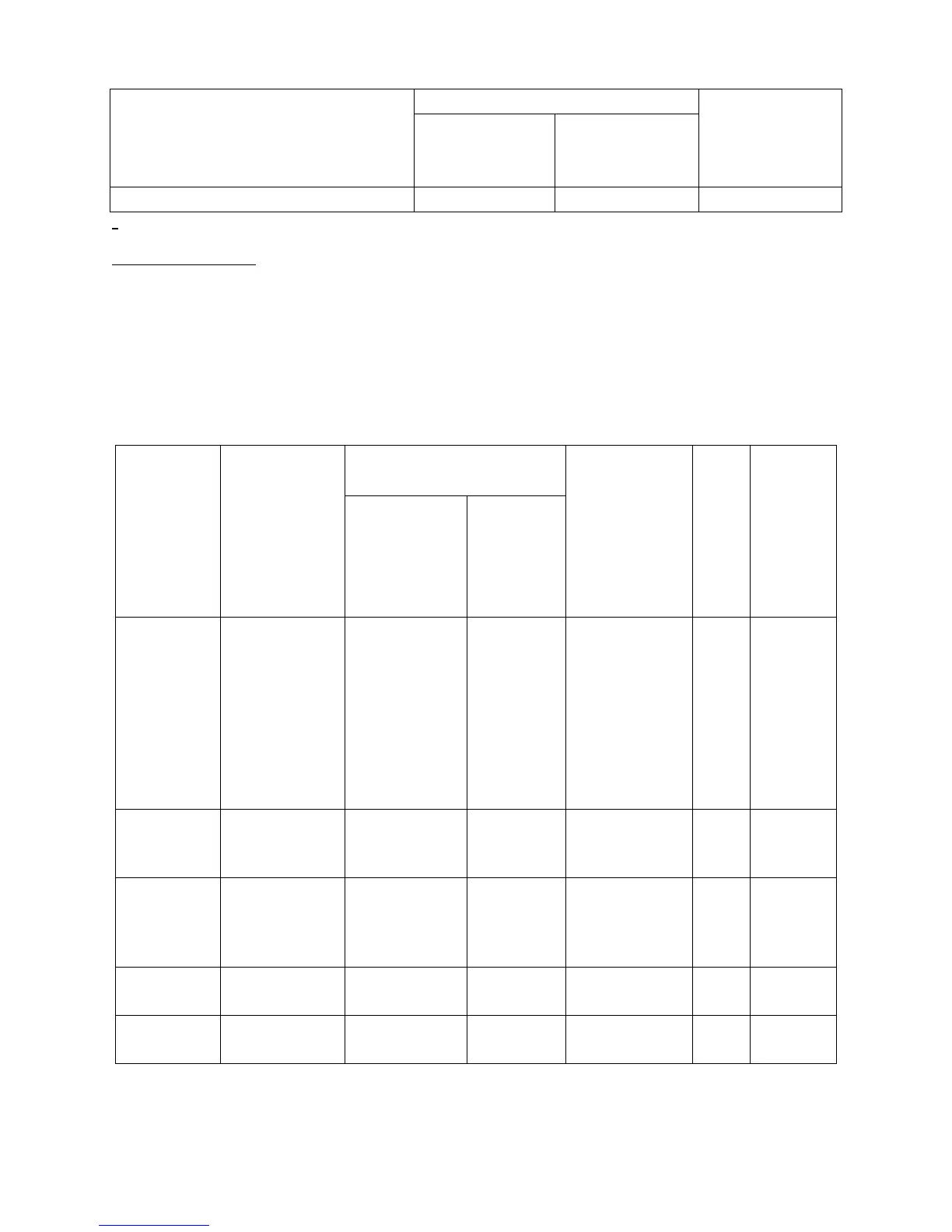
Table 20: Aortic Insufficiency (AI) ≥ Moderate at 1 Year (VI Population)
Quintile Pooled
Proportion
Difference
*
SAPIEN 3 Valve
(N= 1069)
SAVR
(N= 936)
Aortic insufficiency (AI) ≥ moderate 1.6% 0.3% 1.2%
* ATT: average treatment effect on the treated
Secondary Endpoints
The secondary endpoints were examined in a pre-specified order adjusted for the propensity quintiles
using the ATT method. Table 21 summarizes the statistical conclusions on the non-inferiority hypothesis
testing of the five secondary endpoints for labeling that were evaluated using a gatekeeping/hierarchical
multiplicity adjustment procedure to control the overall type I error to 0.05. For each secondary endpoint,
the upper limit of the confidence interval was less than the respective non-inferiority margin. Therefore,
for each of the secondary endpoints for labeling, the SAPIEN 3 valve was non-inferior to SAVR.
Table 21: Secondary Endpoints for Labeling – Gatekeeping/Hierarchical Method
(VI Population)
Pre-
Specified
Order for
Gatekeeping/
Hierarchical
Method
Endpoints
Observed Event Rate
Weighted
Proportion
Difference in
Average
Treatment
Effect on the
Treated
[90% CI]
†
Margin
for Non-
Inferiority
Test
SAPIEN 3 Valve
(N=1069)
PIIA-SAVR
(N=936)
No. 1
Composite of all
death, all
strokes, life
threatening
(disabling)/major
bleeding and
major vascular
complication at
30 days
18.3% 79.4%
-60.5%
[-63.5%, -57.4%]
7.5% Pass
No. 2
Major vascular
complication
through 30 days
5.8% 5.3%
0.3%
[-1.5%, 2.0%]
5.0% Pass
No. 3
Life threatening
(disabling)/major
30 days
14.6% 78.2%
-63.2%
[-66.2%, -60.2%]
5.0% Pass
No. 4
All-cause death
through 30 days
0.9% 3.7%
-2.7%
[-3.9%, -1.5%]
2.5% Pass
No. 5
All stroke
through 30 days
2.6% 6.1%
-3.2%
[-4.7%, -1.6%]
2.5% Pass
†
Two-sided 90% Wald-type confidence interval.
 Loading...
Loading...