25
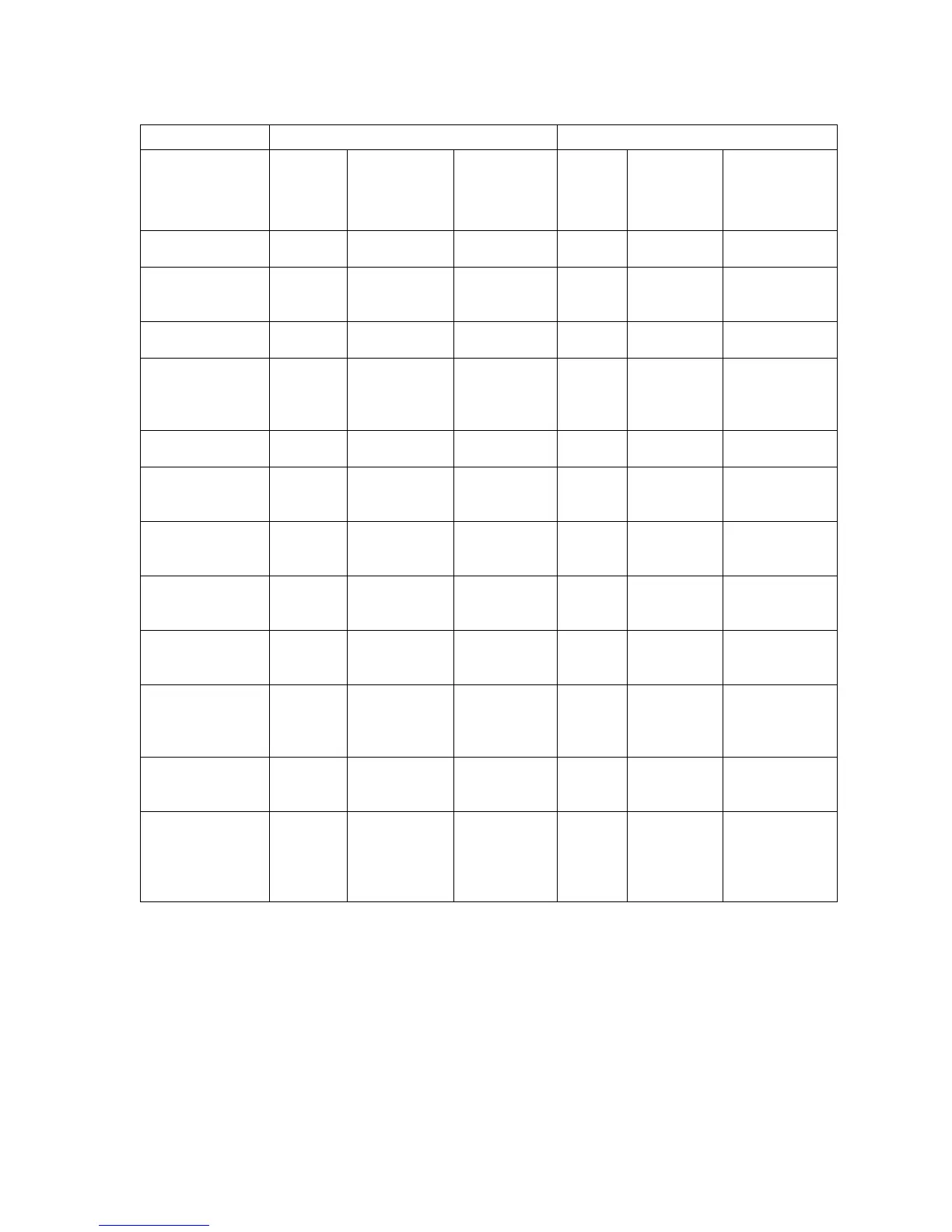
CEC Adjudicated Adverse Events at 1 Year
Outcomes
SAPIEN 3
Valve
Overall
SAPIEN 3
Valve
Transfemoral
Access
Valve
Non-
Transfemoral
Valve
Overall
SAPIEN 3
Valve
Access
SAPIEN 3 Valve
Non-
Transfemoral
Access
cardiovascular
7/102
(6.9%)
2/57
(3.5%)
5/45
(11.1%)
9/102
(8.8%)
2/57
(3.5%)
7/45
(15.6%)
Stroke, n/N (%)
Insufficiency (AI)
≥ Moderate,
3/81
(3.7%)
1/49
(2.0%)
2/32
(6.3%)
1/62
(1.6%)
1/40
(2.5%)
0/22
(0.0%)
Disabling Stroke,
n/N (%)
Infarction,
2/102
(2.0%)
2/57
(3.5%)
0/45
(0.0%)
3/102
(2.9%)
2/57
(3.5%)
1/45
(2.2%)
Complications,
5/102
(4.9%)
1/57
(1.8%)
4/45
(8.9%)
N/A N/A N/A
Injury - Stage III,
0/102
(0.0%)
0/57
(0.0%)
0/45
(0.0%)
N/A N/A N/A
Bleeding Event,
6/102
(5.9%)
3/57
(5.3%)
3/45
(6.7%)
N/A N/A N/A
Requiring
Intervention,
0/102
(0.0%)
0/57
(0.0%)
0/45
(0.0%)
N/A N/A N/A
Endocarditis,
0/102
(0.0%)
0/57
(0.0%)
0/45
(0.0%)
1/102
(1.0%)
0/57
(0.0%)
1/45
(2.2%)
Abnormality
Requiring
Pacemaker,
14/102
(13.7%)
7/57
(12.3%)
7/45
(15.6%)
14/102
(13.7%)
7/57
(12.3%)
7/45
(15.6%)
The composite adverse event rate involving all-cause mortality, all stroke, and AI ≥ moderate at 30 days
for all patients is higher in the S3OUS cohort than PIIS3HR cohort (14.8% vs. 6.8%). This disparity is due
to the composition of the study populations, specifically the S3OUS cohort comprises 44.1% TA/TAo
patients vs. 15.8% TA/TAo patients in the PIIS3HR cohort. Note, the composite adverse event rate at 30
days for TF patients was similar, specifically, 6.0% in the S3OUS cohort and 5.8% in the PIIS3HR cohort.
The K-M estimates for all-cause mortality for all patients, the TF patients, and the TA/TAo patients are
shown in Figure 11.
 Loading...
Loading...