Keep the components in sterilization packaging in
a dry and clean environment.
● Sterility cannot be guaranteed if packaging is
open, damaged or wet.
● Check the packaging and the contra angle
before using it (packaging integrity, no humidity
and validity period).
The instructions provided above have been validated by the manufacturer of the
medical device as being capable of preparing a medical device for use. It remains
the responsibility of the processor to ensure that the processing, as actually
performed using equipment, materials and personnel in the processing facility,
achieves the desired result. This requires verification and/or validation and routine
monitoring of the process. Likewise, any deviation by the processor from the
instructions provided should be properly evaluated for effectiveness and potential
adverse consequences.
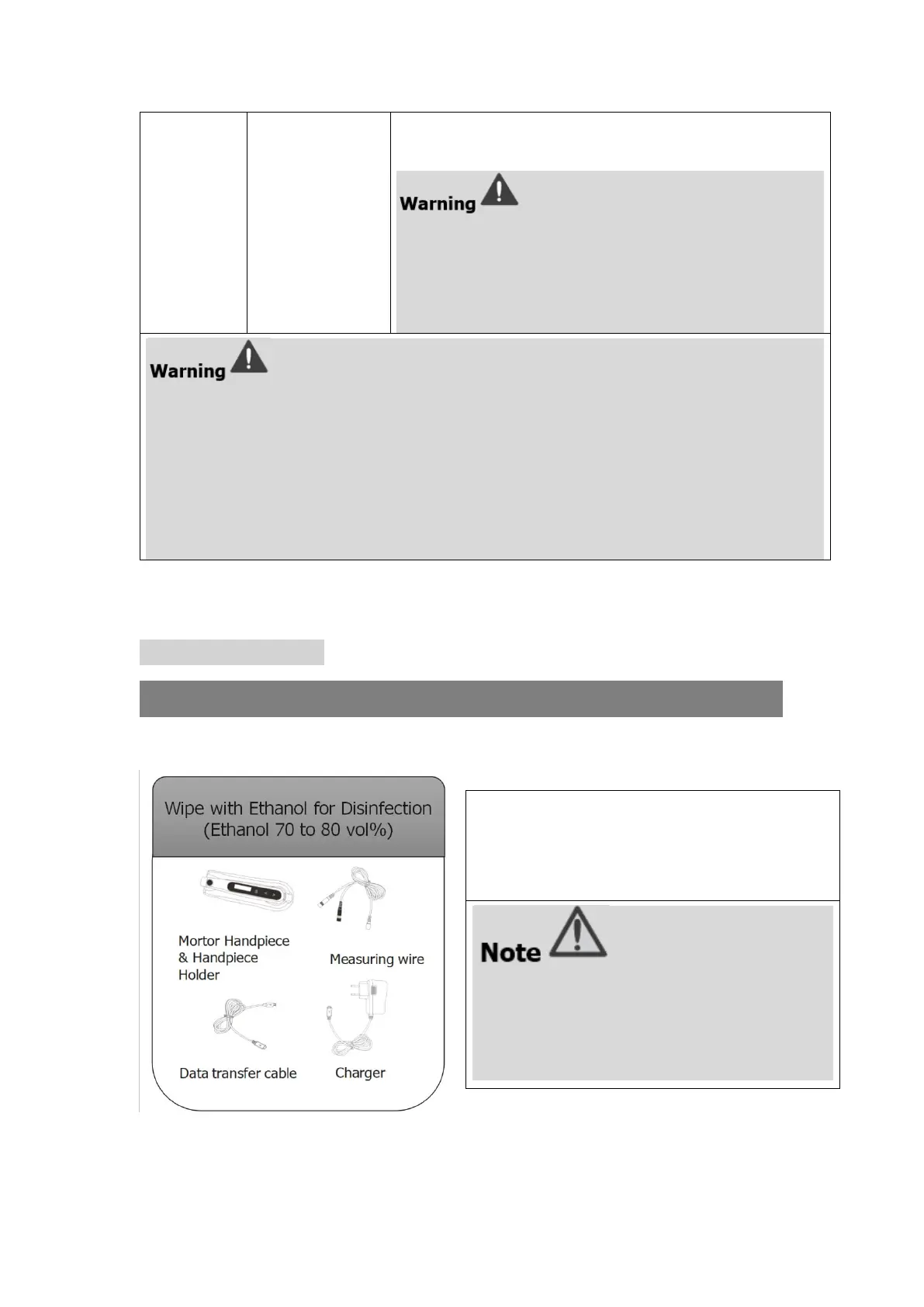
Wipe all the surfaces with a cloth lightly
moistened with Ethanol for Disinfection
(Ethanol 70 to 80vol%) at least 2 min,
repeat for 5 times.
● Do not use anything except Ethanol for
Disinfection (Ethanol 70 to 80 vol%).
● Do not use too much ethanol as it’s going
into machine and damage the components
inside.
 Loading...
Loading...