D. PRODUCT LABELING INFORMATION
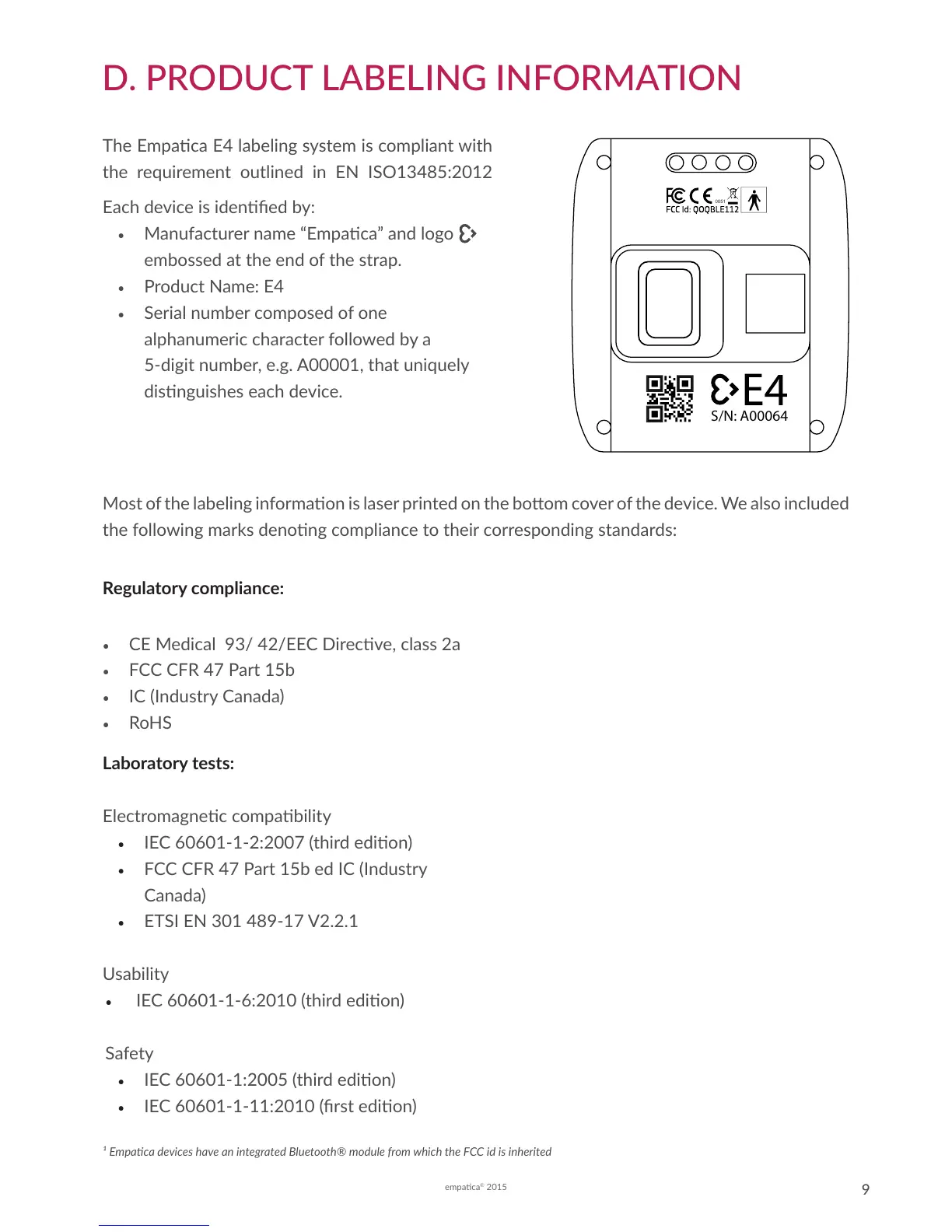
The Empaca E4 labeling system is compliant with
the requirement outlined in EN ISO13485:2012
Each device is idened by:
• Manufacturer name “Empaca” and logo
embossed at the end of the strap.
• Product Name: E4
• Serial number composed of one
alphanumeric character followed by a
5-digit number, e.g. A00001, that uniquely
disnguishes each device.
1 Empaca devices have an integrated Bluetooth® module from which the FCC id is inherited
S/N: A00064
Most of the labeling informaon is laser printed on the boom cover of the device. We also included
the following marks denong compliance to their corresponding standards:
Regulatory compliance:
• CE Medical 93/ 42/EEC Direcve, class 2a
• FCC CFR 47 Part 15b
• IC (Industry Canada)
• RoHS
Laboratory tests:
Electromagnec compability
• IEC 60601-1-2:2007 (third edion)
• FCC CFR 47 Part 15b ed IC (Industry
Canada)
• ETSI EN 301 489-17 V2.2.1
Usability
• IEC 60601-1-6:2010 (third edion)
Safety
• IEC 60601-1:2005 (third edion)
• IEC 60601-1-11:2010 (rst edion)
0051
 Loading...
Loading...