Do you have a question about the Fresenius Kabi CompoSeal Universal and is the answer not in the manual?
Explains the purpose of the operating instructions and defines important symbols used within the document.
Describes the primary uses of the CompoSeal Universal in medical applications.
Explains how the device operates to create a seal using radio-frequency energy.
Lists part numbers and descriptions for the CompoSeal Universal and related accessories.
Specifies the recommended temperature and humidity ranges for storage and operation.
Provides guidance on how to transport and unpack the device safely.
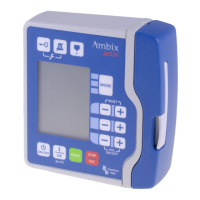
Details the main components and controls of the CompoSeal Universal device with a visual legend.
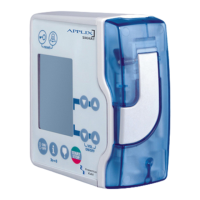
Describes the components of the optional hand sealer and its indicators.
Details specific hazards related to the device's operation, such as sealing connected tubing and electrode contact.
Covers warnings related to liquid contact, extension cables, electrical voltage, and hot surfaces.
Key points to observe before initial start-up, including electrical installation and device suitability.
Step-by-step guide for unpacking, connecting, and powering on the CompoSeal Universal device.
Instructions for inserting tubing, sealing, and removing sealed tubing.
Guidance on connecting, sealing, indicators, and alarms for the optional hand sealer.
Refers to the Technical Manual and notes on contamination affecting sealing quality.
Provides illustrated steps for taking apart the optional hand sealer for cleaning or repair.
Illustrated instructions for lubricating and reassembling the hand sealer after disassembly.
Instructions for removing the sealing cover and cleaning the device's electrodes.
Steps for cleaning the hand sealer, both assembled and disassembled.
Details on the physical characteristics and construction materials of the device and its accessory.
Specifies the maximum outer diameter of the medical PVC tubes that can be sealed.
Information regarding the device's protection against electric shock and ingress of liquids.
Details power requirements, fuses, frequency, and displays of the device's type labels.
Information on fuse types, operating temperature, humidity, and storage conditions.
Guidelines for device disposal, recycling, and explanation of common symbols.
Addresses common problems like sparking and excessive sealing time with recommended actions.
Manufacturer's declaration confirming compliance with relevant EC directives.
| Brand | Fresenius Kabi |
|---|---|
| Model | CompoSeal Universal |
| Category | Medical Equipment |
| Language | English |