GSI
Allegro Tympanometer
User Manual
D-0120695 Rev D 2022-06 Page 51
APPENDIX - USE WITH NON-MEDICAL ELECTRICAL EQUIPMENT
Any person who connects external equipment to signal input, signal output or other connectors
has created a medical electrical system and is therefore responsible for the system complying
with the requirements of clause 16 of IEC 60601-1 (General requirements for basic safety and
essential performance).
If connections are made to standard equipment such as printers and computers, special
precautions must be taken in order to maintain medical safety. The following notes are provided
for guidance in making such connections to ensure that the general requirements of clause 16 of
IEC 60601-1 are met.
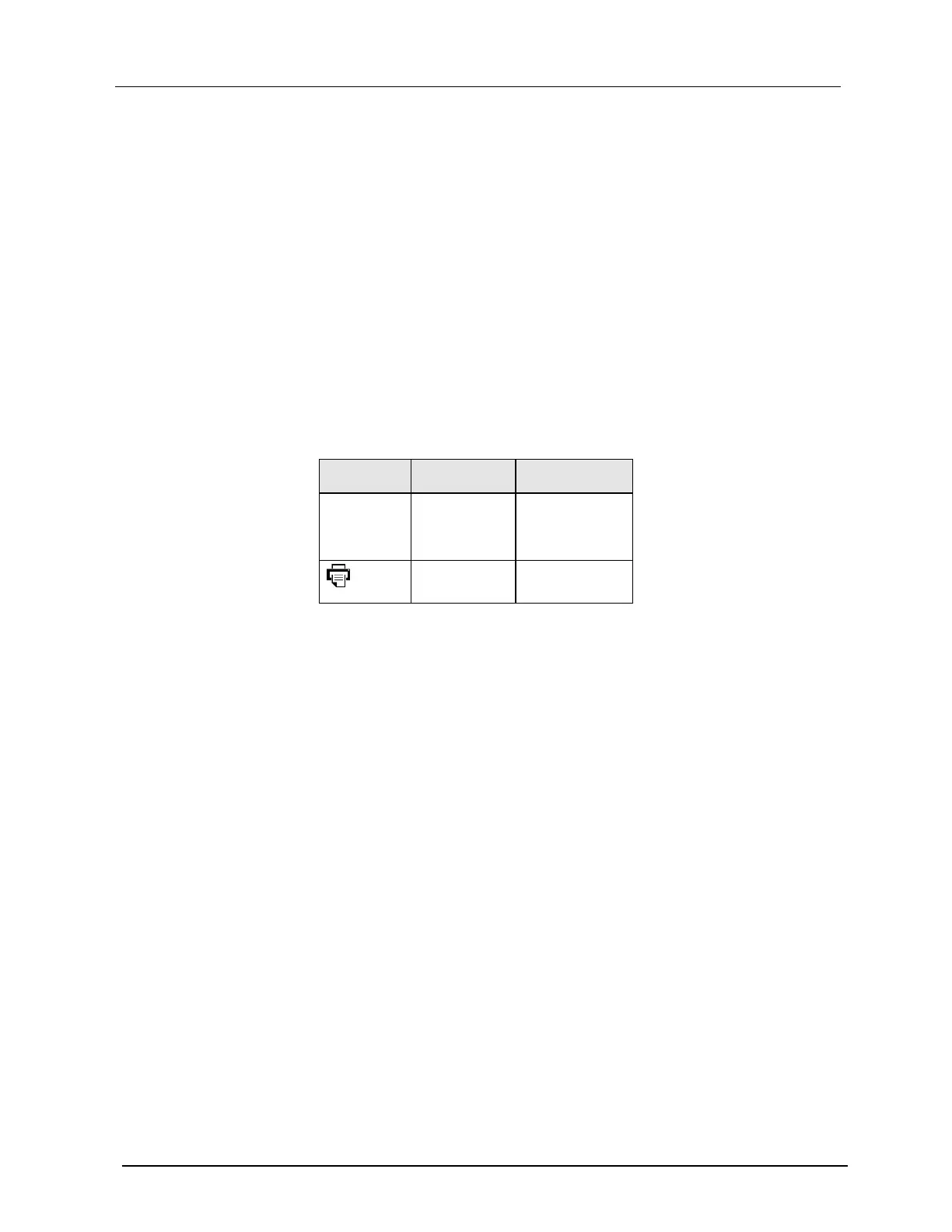
The following signal inputs and outputs on the GSI Allegro tympanometer are electrically isolated
to the requirements of IEC 60601-1:
Type B
These measures are incorporated to reduce any potential hazard associated with the use of
mains-powered equipment connecting to these interfaces.
External equipment intended for connection to signal input, signal output or other connectors,
shall comply with the relevant IEC or international standards (e.g. IEC 60950, CISPR 22 & CISPR
24 for IT equipment, and the IEC 60601 series for medical electrical equipment).
Equipment not complying with IEC 60601 shall be kept outside the patient environment, as
defined in IEC 60601-1 (at least 1.5m from the patient).
The operator must not touch the connected equipment and the patient at the same time as this
would result in an unacceptable hazard.
Refer to GSI at the address given on the front of this user manual if advice is required regarding
the use of peripheral equipment.
 Loading...
Loading...