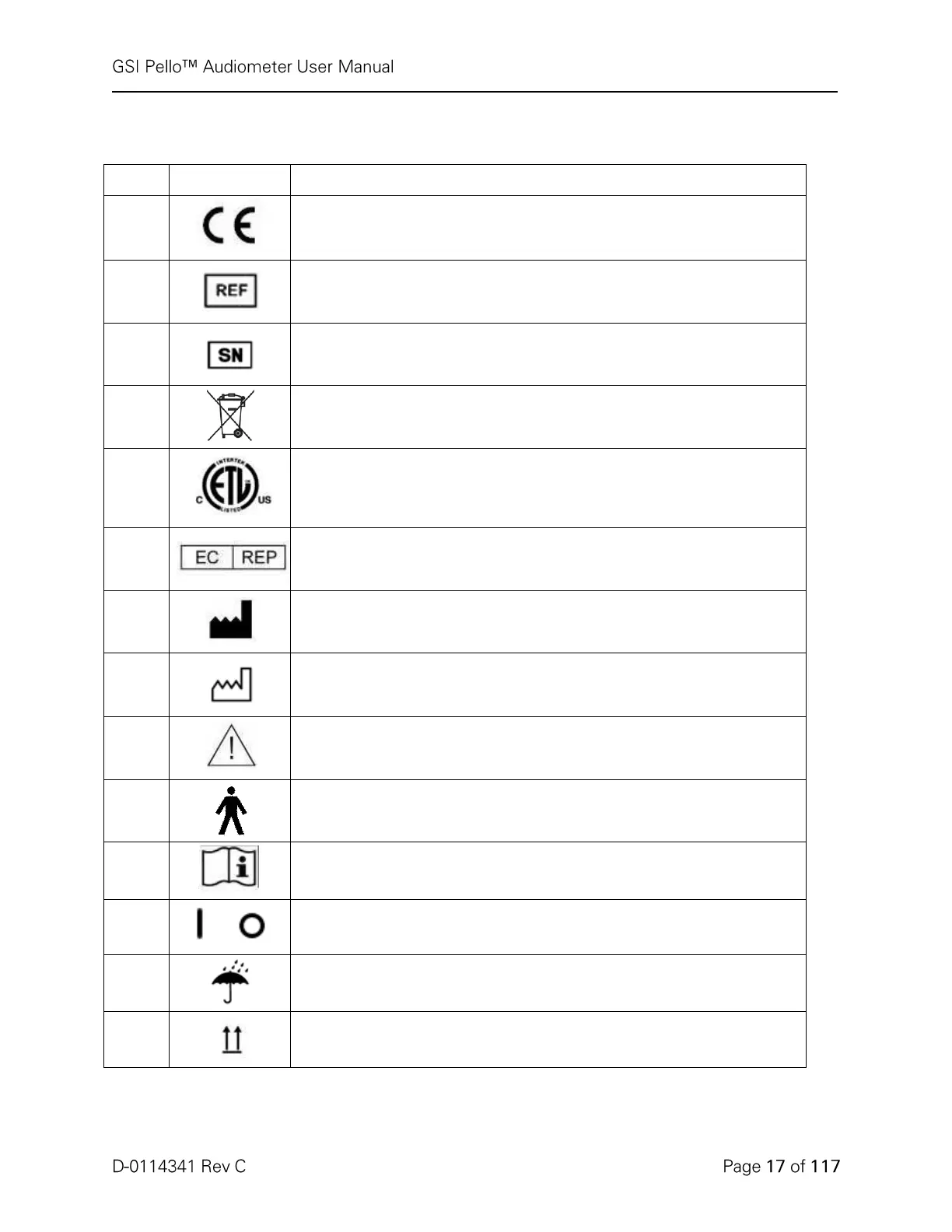
Conforms to European Medical Device Directive 93/94/EEC. Classified
under the Medical Device Directive (93/42/EEC) as a Class IIb device.
GSI Part number and model
Symbol for "SERIAL NUMBER."
Return to Authorized Representative, Special disposal required.
Medical Equipment Classified by Intertek Testing Services NA Inc.
with respect to electric shock, fire, and mechanical hazards only, in
accordance with UL 60601-1. Classified under the Medical Device
Directive (93/42/EEC) as a Class IIa device.
Symbol for “European Representative.”
Symbol for “Manufacturer.”
Symbol for “Date of Manufacture.”
Attention, consult accompanying documents.
Type B Patient Applied Part according to IEC 60601-1.
Consult Operating Instructions.
On/Off - Next to power mains.
 Loading...
Loading...